Table of Contents
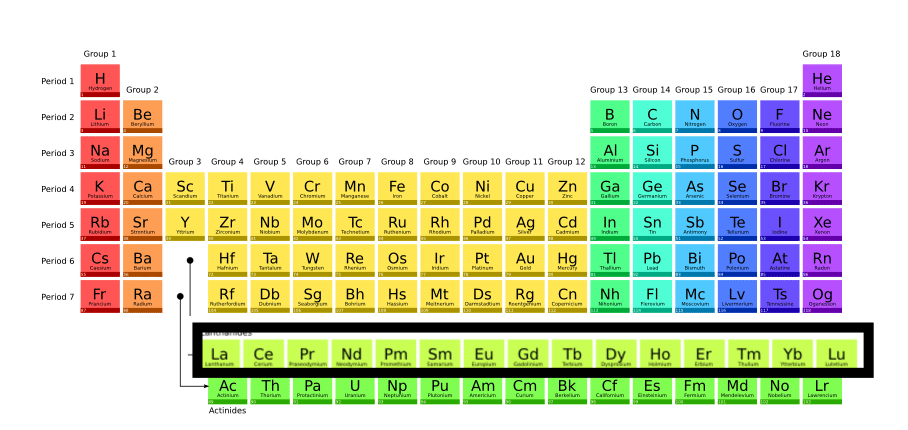
ToggleLanthanides are those elements in which the last electron enters into anti-penultimate 4f sub-shell. These are the elements from Ce58 to Lu71 that lie in the 6th period of the periodic table and are called f-block elements since the differentiating electron (last electron) in the atom of these elements enters the f sub-shell belonging to anti-penultimate shell [(n-2)th shell].
Electronic Configuration of Lanthanides
The general electronic configuration of the Lanthanide element is
[Xe] 4f1-14 5d10-1 6s2
Since the inner electron level, 4f orbitals are successively filled from (4f1 to 4f14) though there are outer incompletely filled electron levels, these elements are called inner transition elements and the series are called inner transition series.
The electronic configuration of lanthanide elements are
| Element | Symbol | Atomic number | Electronic structure of atoms | Electronic structure of M3+ | Oxidation states |
| Lanthanum | La | 57 | [Xe] 5d1 6s2 | [Xe] | +III |
| Cerium | Ce | 58 | [Xe] 4f1 5d1 6s2 | [Xe] 4f1 | +III, +IV |
| Praseodymium | Pr | 59 | [Xe] 4f3 6s2 | [Xe] 4f2 | +III |
| Neodymium | Nd | 60 | [Xe] 4f4 6s2 | [Xe] 4f3 | +III |
| Promethium | Pm | 61 | [Xe] 4f5 6s2 | [Xe] 4f4 | +III |
| Samarium | Sm | 62 | [Xe] 4f6 6s2 | [Xe] 4f5 | +III |
| Europium | Eu | 63 | [Xe] 4f7 6s2 | [Xe] 4f6 | +II, +III |
| Gadolinium | Gd | 64 | [Xe] 4f7 5d1 6s2 | [Xe] 4f7 | +III |
| Terbium | Tb | 65 | [Xe] 4f9 6s2 | [Xe] 4f8 | +III |
| Dysprosium | Dy | 66 | [Xe] 4f10 6s2 | [Xe] 4f9 | +III |
| Holmium | Ho | 67 | [Xe] 4f11 6s2 | [Xe] 4f10 | +III |
| Erbium | Er | 68 | [Xe] 4f12 6s2 | [Xe] 4f11 | +III |
| Thulium | Tm | 69 | [Xe] 4f13 6s2 | [Xe] 4f12 | +III |
| Ytterbium | Yb | 70 | [Xe] 4f14 6s2 | [Xe] 4f13 | +II, +III |
| Lutetium | Lu | 71 | [Xe] 4f14 5d16s2 | [Xe] 4f14 | +III |
Although La lacks f electrons, its properties are comparable to those of the lanthanides, therefore it is classified and studied alongside them.
The 14 elements from Cerium to Lutetium are expected to be formed by adding 1,2,3…….14 electrons to the 4f-level. In most electrons, however, moving single 5d electrons into the 4f level is energetically advantageous, but not in Ce, Gd, or Lu. Gd has a 5d1 configuration because it provides a half-filled 4f level, which provides extra stability. Because the f shell is already occupied, Lu has a 5d1 configuration. The lanthanides are distinguished by the fact that all of the metals have the same +III oxidation state.
Oxidation States of Lanthanides
All lanthanides exhibit the most common and stable oxidation state of +3. This is because the sum of each lanthanide’s first three ionization energies is low, allowing them to easily form their trivalent states. Other oxidation states +2 and +4 do exist for some lanthanides, however, they are less stable than +3. Hence oxidation state +2 and +4 occurs when they lead to
- noble gas configuartion e.g. Ce+4 (f0)
- a half-filled f-shell e.g. Eu2+ and Tb4+ (f7)
- a completely filled f-shell e.g. Yb2+ (f14)
Furthermore, elements that are near to these states also possess (+II) and (+IV) states. Examples: Sm2+ and Tm2+ have f6 and f13 arrangements, respectively, while Pr4+ and Nd4+ have f1 and f2 configurations.
Magnetic properties of Lanthanides
Since the elements like La3+ and Ce4+ have noble gas configuration (f0), and Lu3+ has a completely filled f-shell configuration (f14), these elements are diamagnetic due to the absence of unpaired electrons. All other f states are paramagnetic as they have unpaired electrons.
The magnetic moment of the transition elements can be calculated by using the formula:
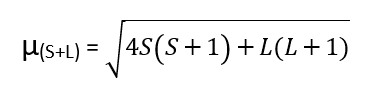
where, µ(S+L)= magnetic moments in Bohr magnetons, S = resultant spin quantum number, and L= resultant orbital momentum quantum number.
Solubility of Lanthanides
Many salts follow the pattern of group 2 elements in terms of solubility. Thus, chlorides and nitrates are soluble in water, whereas oxalates, carbonates, and fluorides are not; however, suplhates are. Many lanthanides combine with group 1 or ammonium salts to form double salts. Because these double salts crystallize well, they’ve been utilized to separate the lanthanides.
Color and Spectra of Lanthanides
The lanthanides ions absorb light in the near-ultraviolet and visible ranges. The color of these elements is due to the presence of unpaired f-electrons which take part in the f-f transition. All the Ln3+ except Lu3+ are coloured, both in their salts and aqueous solutions. Crystal field splitting has a significant role in the spectra of transition metals.
| Number of 4f electrons | Colour | Number of 4f electrons | Colour | ||
| La3+ | 0 | Colourless | Lu3+ | 14 | colourless |
| Ce3+ | 1 | Colourless | Yb3+ | 13 | colourless |
| Pr3+ | 2 | Green | Tm3+ | 12 | Pale green |
| Nd3+ | 3 | Lilac | Er3+ | 11 | Pink |
| Pm3+ | 4 | Pink | Ho3+ | 10 | Pale yellow |
| Sm3+ | 5 | yellow | Dy3+ | 9 | Yellow |
| Eu3+ | 6 | Pale pink | Tb3+ | 8 | Pale pink |
| Gd3+ | 7 | colourless |
Uses of Lanthanides
- Used as catalysts in the production of petroleum and synthetic products.
- Used for making steel alloys.
- Lanthanide oxides are used to color ceramics and glasses and are also used as phosphors on television screens.
- Cerium oxide (CeO2) is used to polish glass.
- Samarium is used in laser lamps.
- Lanthanide-cobalt alloys are used for making permanent magnets.
- Used for the removal of Sulphur and oxygen impurities.
Lanthanides Video
FAQs/MCQs
What are the lanthanides?
Lanthanides are those elements in which the last electron enters into the anti-penultimate 4f sub-shell.
How many elements are in the lanthanide series?
There are 14 elements in the lanthanide series that range from Ce58 to Lu71
Are lanthanides metals?
Lanthanides are metallic elements occupying the position of 57th to 71th of sixth period in the modern periodic table
lanthanides and actinides
Actinides are those elements in which last electron enters 5f sub-shell.
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.






