Table of Contents
ToggleDiastereomers are stereoisomers that have the same molecular formula but are not mirror images of each other. These are non-superimposable, and may or may not be optically active compounds. The phenomenon by which stereoisomers exhibit such behaviors is called Diastereoisomerism. The stereoisomers that have one stereocenter, and are non-superimposable mirror images of each other are enantiomers, while those having two or more than two stereocenters having non-superimposable non-mirror images of each other are diastereomers. The maximum number of stereoisomers that can exist is equal to 2n, where n is the number of chiral centers. For the compounds that have 2 chiral centers, there could be as many as 4 stereoisomers
Unlike enantiomers, diastereomers differ in physical and chemical properties. Since the diastereomers are anisometric to each other, they have different shapes, the energy of activation, rate of reactions, boiling points, melting points, specific rotation, dipole moment, and so on. These can be separated by chromatography, fractional crystallization, fractional distillation, etc.
Conditions for Diastereosiomers
- Must have two or more than two stereocenters.
- Non-superimposability
- Non-mirror images of each other
Characteristics of Diastereoisomers
- Diastereomers exhibit different physical ( melting points, boiling points, densities, solubilities, refractive indices, dielectric constants, and specific rotations) as well as chemical properties ( rate of reactions).
- Other than geometrical isomers, diastereomers may or may not be optically active.
- Since enantiomers have the same physical properties, it is very difficult to separate them. However, diastereomers can be separated by fractional crystallization, fractional distillation, chromatography, etc.
Examples of Diastereomers
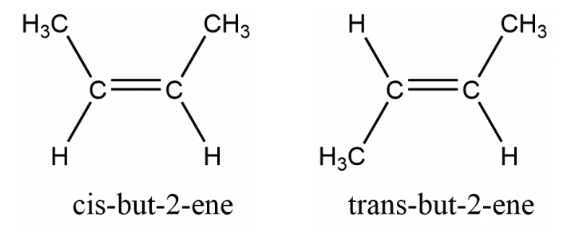
cis-trans isomers are said to be diastereomers since they are not mirror images of one another.
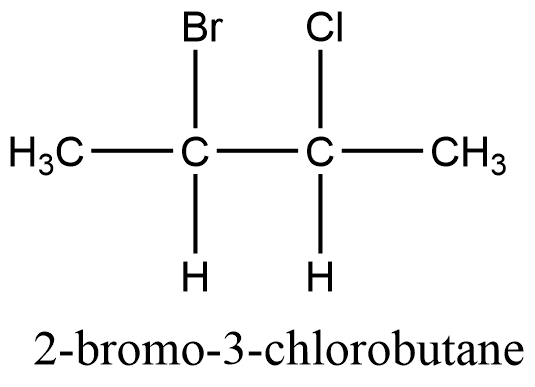
Let’s take an example of 2-Bromo-3-chlorobutane, there are 2 chirality centers, C2 and C3.
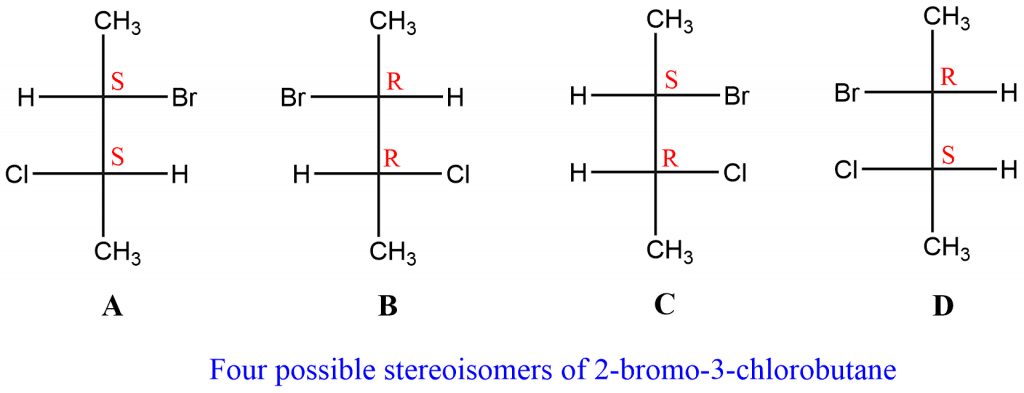
The total number of possible stereoisomers for this compound is four. In the stereoisomers illustrated below, A & B, and C & D are enantiomers since they are non-superimposable mirror images of each other. The stereoisomers A & C, and B & D are diastereoisomers as they are not mirror images of each other.
Diastereomers Video
FAQs/MCQs
Diastereoselectivity
The preference for the formation of one or more diastereomers in an organic reaction is known as diastereoselectivity.
Enantiomers
Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other.
Do diastereomers have a plane of symmetry?
Yes, diastereomers have a plane of symmetry with two chiral centers.
Are diastereomers optically active?
Other than geometrical isomers, diastereomers may or may not be optically active.
What are diastereomers?
Diastereomers are stereoisomers that have the same molecular formula but are not mirror images of each other.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






