Table of Contents
ToggleDuring the synthesis of desired compounds in the laboratory, the purification process is an essential step to get that product in pure form. A chemist can use various purification techniques such as crystallization, filtration, distillation as well as chromatographic techniques.
Before discussing flash chromatography, it is necessary to explain column chromatography in brief because flash chromatography is an advanced modification of traditional column chromatography.
In traditional column chromatography, a crude reaction mixture or simply sample mixture is applied on the top of a glass column packed with a bed of silica gel. Then, a suitable mobile phase passes through the vertical column of silica gel which acts as a stationary phase. Thus, the separation of the mixture into its component takes place.
Remember one point!!! In traditional column chromatography, the mobile phase moves down the vertical column due to the effect of gravity, we do not apply external pressure. Thus, the mobile phase is gravity-fed, meaning the mobile phase moves down due to gravity.
What is flash chromatography?
Flash chromatography is sometimes called Flash column chromatography. This term was first coined by W. Clark still and co-workers at Columbia University to describe the separation process using a gas-pressurized solvent reservoir.
Principle of Flash column chromatography
In this chromatographic method, compressed gas is used to put pressure on the solvent(mobile phase) reservoir to accelerate the flow rate of the mobile phase so that superior chemical separation is achieved in less time than traditional gravity-based column chromatography.
The principle of flash chromatography is basically similar to that of column chromatography. The chemicals in the mixture are separated based on their affinity for the mobile phase and stationary phase, which causes them to migrate through the column at various rates and comes out from the bottom at different time.
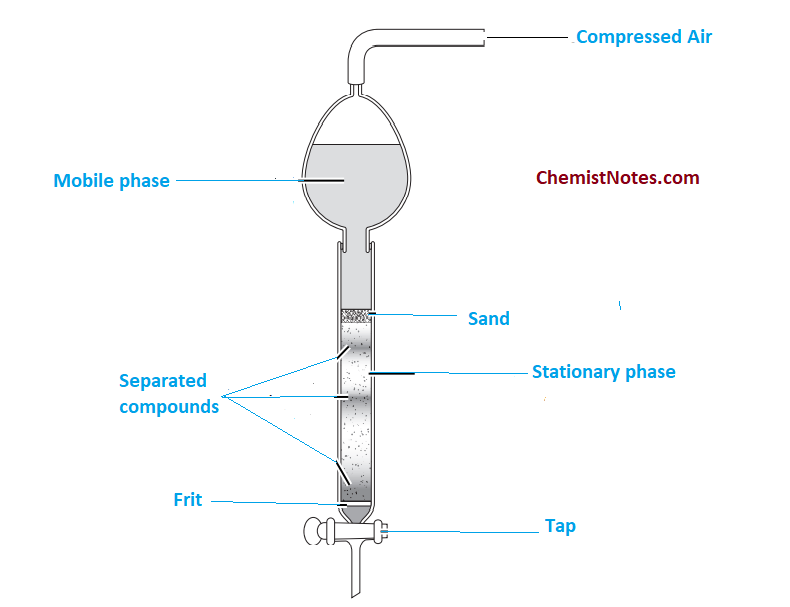
Flash column chromatography equipment
It is an easy and simple purification technique that requires minimal method development. Let’s discuss the basic components of it
- Pump system
- Mobile phase
- Mobile phase modifier
- Stationary phase
- Columns
- Cartridges
- Detector

Pump system
The pump system consists of a pump controller and a vacuum pump. The pump controller regulates the pressure range required for optimum separation. The vacuum pump helps to accelerate the flow of the mobile phase from the reservoir.
Mobile phase
The choice of mobile phase depends on the polarity of the mixture to be separated and the type of stationary phase being used.
- If we use normal-phase silica gel as a stationary phase, the solvent system must have lower polarity. For example; dichloromethane/methane, hexane/ethyl acetate, hexane/ether, etc.
- If we use reversed-phase silica gel as a stationary phase, the solvent system must have high polarity. For example; water/isopropanol, water/acetonitrile, etc.
Moreover, while choosing a suitable mobile phase, the chemist must consider the solubility of the mixture to be separated. It means, the mobile phase must be able to dissolve the sample component completely without forming precipitation. However, certain low-polarity mobile phases may precipitate an oily mixture during solution before putting the sample on the column or after loading the sample on the top of the column. To solve this issue, we must choose a solvent with enough polarity.
Then, how does a chemist find out the best mobile phase? In order to determine the best solvent system for optimal separation, they first perform analytical trials with the TLC technique. In this process, chemists will choose only those solvent that moves the mixture components efficiently so that the Rf value is at least equal to 0.25 and keeps undesired components to a distance of at least 0.2 Rf value.
Some commonly used solvents as mobile phases in the chromatography and corresponding polarity value are listed below:
| Solvents | Polarity |
| Hexane | 0.06 |
| n-Heptane | 0.20 |
| Toluene | 2.40 |
| Methyl Chloride(DCM) | 3.40 |
| Tetrahydrofuran | 4.20 |
| Ethanol | 4.30 |
| Ethyl acetate | 4.30 |
| 1-propanol | 4.30 |
| Acetonitrile | 6.20 |
| Methanol | 6.60 |
| Water | 10.28 |
Sometimes, a mixture of two solvents one having higher polarity and the other having lower polarity is used as the mobile phase to enhance better separation. For example, Hexane/Ethyl acetate(1:1), dichloromethane/methanol(95:5), etc.
Note: What are the meanings of the terms solvent system strength and solvent selectivity? The first one represents the ability of the solvent to migrate all compounds simultaneously on the column. But, selectivity indicates the solvent’s ability to migrate one specific compound differently from another.
Mobile phase modifier
When molecules have acidic or basic groups, they may interact with the residual surface silanol groups on chromatographic support. Such interaction results in peak tailing, which is not considered good in chromatographic separation.
In order to reduce peak tailing, a chemical regent is added in a very low concentration, typically 1% or less. Such a reagent is called a mobile phase modifier. Mobile phase modifier sharpens peaks and thus improves the resolutions in separations of basic or acidic compounds.
Examples of common mobile phase modifiers are Triethylamine, acetic acid, ammonium hydroxide, and trifluoroacetic acid.
Stationary phase
The stationary phase is selected on the basis of the nature of the organic compounds to be separated. In other words, the polarity of organic molecules and the functional groups present in them are two major factors that guide the selection of the best stationary phase. The first stationary phase used in flash chromatography was Silica gel but later other stationary phases including reverse phase C18, alumina, and ion exchange resin were used. However, a normal phase or a reversed-phase silica gel is used as the stationary phase.
- If the sample has a low polarity, we can use normal phases, reverse phase, neutral alumina, etc. as stationary phase
- If the sample has a high polarity, we can use C18 or the cyano stationary phase.
- If the sample has basic functional groups, we can use C18, normal phase, basic alumina, and strong cation exchange(SCX).
- If the sample has acidic functional groups, we can use normal phase, C18, acidic alumina, neutral alumina, and strong anion ethers.
- If the sample is acid sensitive, use neutral alumina or diol, or cyano stationary phase.
- If the samples are charged, use C18 or cyano-based stationary phase.
Types of Elution/Techniques of mobile phase
Isocratic elution
In isocratic elution, the composition of the mobile phase remains the same throughout the experiment.
- The mobile phase may be a single solvent or a mixture of two solvents.
- mobile phase composition does not change during the separation.
- This technique is used by most classical flash chromatography.
Gradient elution
In gradient elution, mobile phase composition is varied during chromatographic separation. Gradient methods are of three types viz. Stepped gradient, Linear gradient, and mixed gradient. This technique offers the following advantages:
- it needs very short elution times
- it offers high purity
- it offers a greater sample loading capacity
- it has a greater repeatability
Flash chromatography columns
In general, there are two types of columns that can be used in flash chromatography.
- Manually-packed Columns: These columns are prepared by loading suitable stationary phases in the glass columns. These hand-packed columns may not be perfectly packed and hence, decreases the resolution.
- Pre-packed columns: Manually packing of columns is a very tedious process, thus pre-packed columns are also available commercially in the market in various sizes. The effectiveness of compound purification is increased by using pre-packed columns, which also provide higher productivity and repeatability.
The usage of the pre-packed columns purifies compounds quickly as it saves the time required to prepare columns. On the other hand, the pre-packed columns are safer to utilize than the manually packed columns because users are not exposed to silica dust.
Flash chromatography cartridges
Cartridges are cylindrical pipe-like devices that are used to introduce the sample onto the columns These cartridges are mainly used for low-solubility samples in automated flash chromatography.
There are mainly two types of cartridges commercially available in the market.
- Empty solid-load cartridges
- Pre-packed solid load cartridges
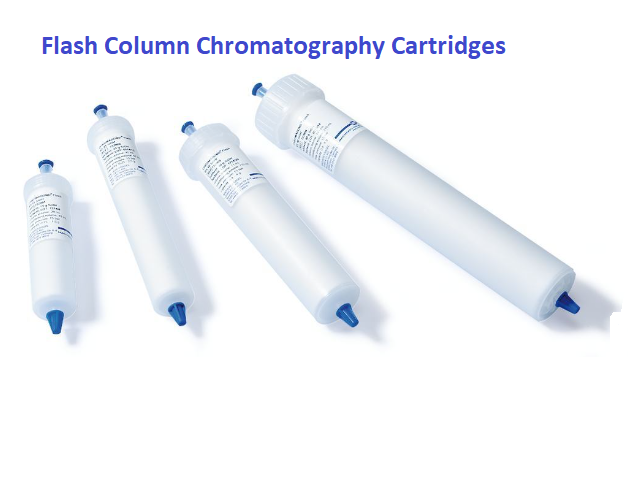
Empty solid load cartridge
These cartridges can be used with various adsorbent materials, but generally, silica gel is used.
- First, the sample to be purified is dissolved in a suitable solvent and powder silica gel is mixed with it. Then, with the help of a rotatory evaporator solvent is removed leaving behind the sample coated with the silica gel.
- After then, the sample coated with silica gel is poured into an empty cartridge and loaded into the system.
Not only silica gel, other various materials such as Celite, diatomaceous earth, boiling chip, etc can be used as an adsorbent in cartridges. These cartridges are connected directly to the column which removes the chances of contamination.
Pre-packed solid-load cartridges
These are ready-made cartridges available in the market and people prefer these cartridges over empty cartridges because the packing process as described above of cartridges seems tedious. Using these pre-packed solid cartridges, samples can be loaded very fast.
- First, the sample is dissolved in suitable solvents and then the dissolved sample is applied to the pre-packed cartridges.
- When the adsorbent absorbs the dissolved sample completely, the wet cartridge is completely dried with a high vacuum pump before being put into the system.
Detection techniques in Flash chromatography
Before the introduction of automation, chemists used to collect all the fractions, and these fractions are then tested by using TLC plates. But after automation in flash chromatography, various detectors are set up in the instruments to detect the separated compounds.
- UV-vis detector
- Refractive index detector
- Fluorescence detector
- Evaporative light scattering detector(ELSD)
Most of the compounds are UV active, so a UV detector is the most commonly used detector in Flash Chromatography.
Flash column chromatography procedure
After the selection of a suitable stationary phase and mobile phase by analytical trials using TLC, the Chemist loads the sample onto the columns. The sample loading step is a very challenging step as the state of the sample determines the way of loading.
There are different ways of sample-loading techniques for both manual and automated chromatography equipment.
Sample loading technique for manual flash chromatography
- If the sample is liquid, it is pipetted slowly inside the columns. Then, the selected mobile phase for optimal resolution is slowly introduced from the top of the columns.
- If the sample is solid, it is dissolved in a suitable polar solvent and silica gel powder is added. Then, a rotatory evaporator is used to evaporate the sample, leaving the sample coated on the silica gel. This silica sample is then placed on the top of the column.
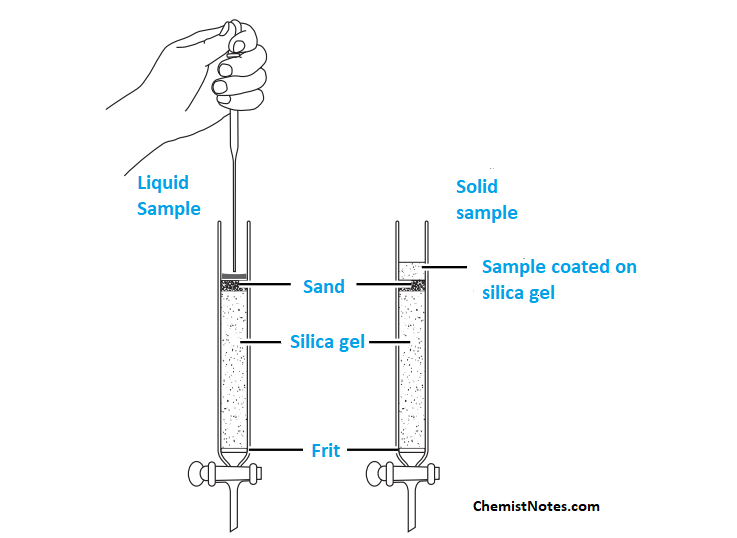
Sample loading technique for automated flash chromatography
- Using syringe injection: It is a very common and simple technique for sample loading and it requires the sample to be dissolved in the mobile phase first. This method allows the equilibration of the column, which is required for efficient separation.
- Using cartridges: These are mainly used for the introduction of low-solubility samples onto the column.
Flash chromatography vs column chromatography
The difference between flash chromatography and column chromatography is that there is no usage of compressed air for the mobile phase in column chromatography. Therefore, separation time is more for column chromatography and, due to the application of air pressure, flash chromatography offers high resolution of separation in a very short time.
Advantages and disadvantages of Flash chromatography
The advantages and disadvantages of manual glass flash column chromatography are listed below:
Advantages
- Low cost
- It is possible to employ stepped gradient and isocratic solvent elution.
- The chemist totally regulates the introduction of stationary phase and packing techniques.
Disadvantages
- Low resolution
- It is difficult and time-consuming to separate complicated crude reaction mixtures.
Automated flash chromatography
In recent years, this technique has gained more attraction due to the introduction of the automation system. Automated flash chromatography offers the following advantages over manual glass-column flash chromatography.
- This technique offers full automation from sample injection to compound collection. Thus it removes the necessity for extra manpower and tedious process for method development.
- The time of separation is dramatically reduced and hence it saves time.
- It purifies a wide range of sample sizes.
- It reduces the usage of solvent(mobile phase) and purification time without compromising the resolution of separation.
Application of Flash column chromatography
It is now one of the most widely used methods for purifying pharmaceutical intermediates as well as final products. Moreover, it is commonly used in the study of natural products. For examples;
- Isolation and separation of catechin from green tea extracts.
- Purification of fatty acids
- Purification of steroids






