Table of Contents
ToggleChelating agents are the polydentate ligand that form the chelates. Chelating agent with three, four and six donor atoms are known and are termed tridentate, tetradentate, and hexadentate ligand. Chelating agents must possess at least two donor groups per molecule; that is, the ligands should be polydentate. These donor groups must be situated in the molecule in such a way that they permit the formation of a ring with the metal atom without any strain. The donor atoms should be sterically capable of coordinating to the same metal to form a chelate.
A ligand which has more than one point of attachment to the central metal ion in a complex is called polydentate ligand. A ligand may occupy more than one coordination site in certain complexes. As a result, the central metal is linked to more than one atom in the ligand. For example, ethylenediamine forms a complex with copper ions.
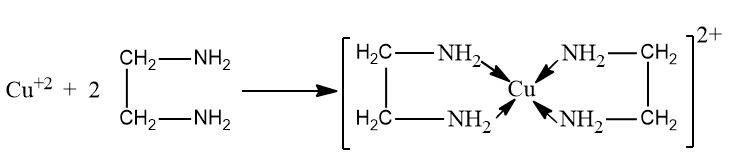
In this complex the copper is surrounded by four -NH2 groups. Thus each ethylenediamine molecule is bonded to the copper in two places. For this reason ethylenediamine is called a bidentate group or ligand. A ring structure is thus formed and such ring structures are called chelates.
Examples of chelates
Chelated complexes are more stable than similar complexes with unidentate ligands, as dissociation of the complex involves breaking two bonds rather than one. Some examples of metal chelates are;
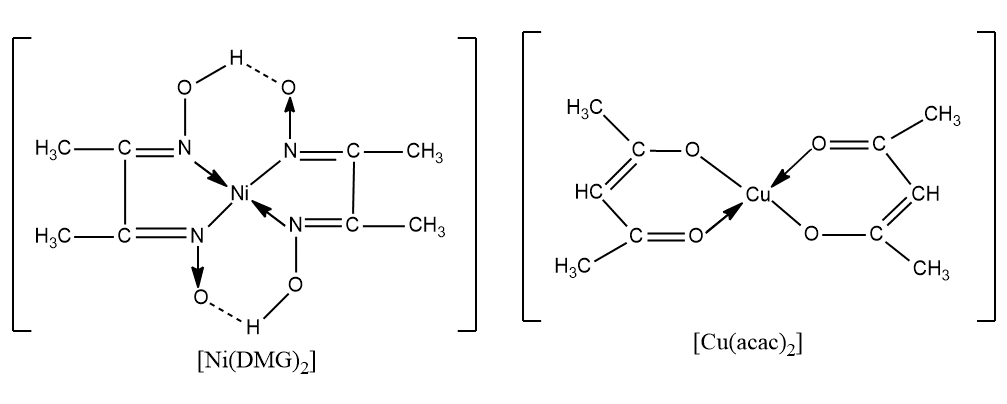
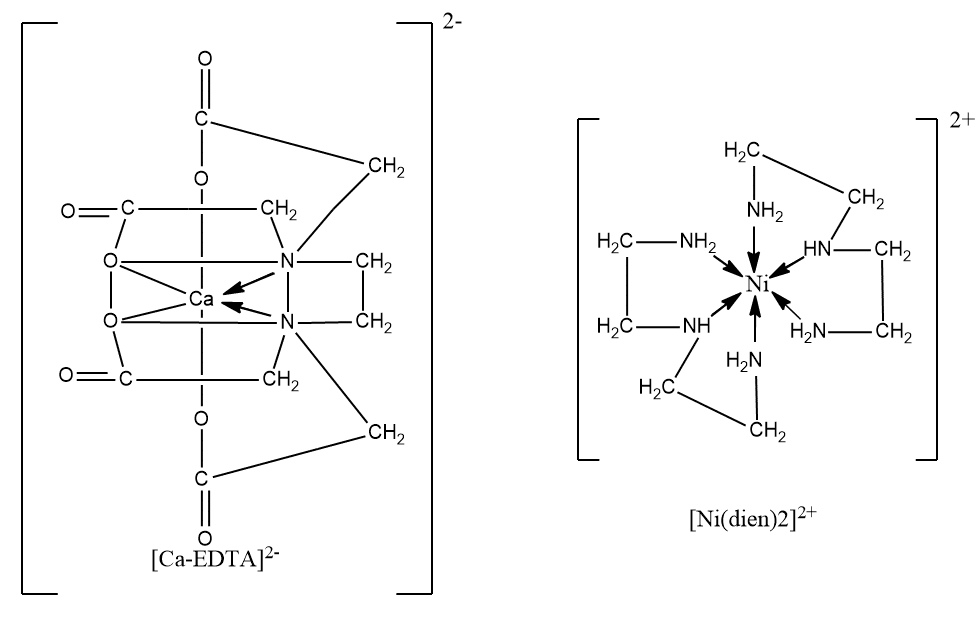
several chelate compounds are of biological important.
Chelate effect
The chelate effect is thermodynamically related to the reaction’s entropy change. When a solvated metal ion combines with a chelating agent, the chelating agent substitutes the solvent molecules in the coordination sphere of the metal ion. for example;
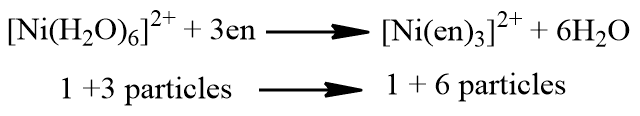
This chelation process results in an increase of three particles (7 – 4 =3). The replacement of the monodentate ligand by chelating agents always has this effect. This increase the number of particles results in an increase in the randomness of the system. This in turn, an increase in the degree of randomness of the system leads to greater stability of the complex.
The stability of a chelate complex is higher than that of a non-chelate complex of the same metal ion.
Applications of Chelating Agents
- Chelation treatment is the use of chelating agents to detoxify toxic metals like mercury, arsenic, and lead by converting them to a chemically inert state that may be expelled without further interaction with the body.
- The US Food and Drug Administration authorized this treatment in 1991. Chelating agents can be helpful in cases of heavy metal toxicity, but they can also be deadly. The use of disodium EDTA instead of calcium EDTA has resulted in hypocalcemia-related deaths.
- Almost all biochemicals have the ability to dissolve certain metal cations. Proteins, polysaccharides, and polynucleic acids are thus effective polydentate ligands for a wide range of metal ions.
- Organic compounds such as the amino acids glutamic acid and histidine, organic diacids such as malate, and polypeptides such as phytochelatin are also typical chelators. In addition to these adventitious chelators, several biomolecules are specifically produced to bind certain metals.
- Almost all metalloenzymes contain metals that have been chelated, generally to peptides, cofactors, or prosthetic groups. The porphyrin rings in hemoglobin and chlorophyll are examples of chelating agents.
- Many microbial species produce water-soluble pigments that serve as chelating agents, termed siderophores. For example, species of Pseudomonas are known to secrete pyocyanin and pyoverdin that bind iron. Enterobactin, produced by E. coli, is the strongest chelating agent known.
FAQs
What is chelating agent?
Chelating agents are the polydentate ligand that form the chelates.
Is ethylenediamine a chelating agent?
Ethylenediamine is a bidentate chelating ligand.
What does a chelating agent do?
Chelation therapy is the use of chelating chemicals to detoxify toxic metals like mercury, arsenic, and lead by converting them to a chemically inert state that may be expelled without further interaction with the body.






