Table of Contents
ToggleWhat is acetone?
Acetone (or propanone or dimethyl ketone) is the simplest and most important aliphatic ketone with the chemical formula (CH3)2C=O. It is formed during the destructive distillation of wood and is found in the ‘Wood spirit’. It is highly soluble in a wide range of organic compounds. It is a colorless, extremely volatile, and flammable liquid with a distinctive pungent odor.
Acetone is created and excreted in the human body as part of regular metabolic processes. It is generally found in the blood and urine. It is produced in greater amounts in those with diabetes patients. On the other hand, is an irritant that causes mild skin irritation and moderate to severe eye discomfort. Like many other solvents, it may depress the central nervous system at high vapor concentrations.
Preparation of Acetone
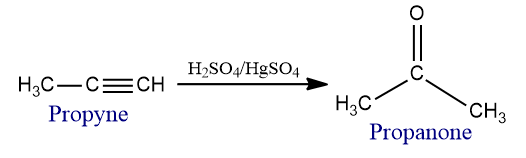
- Acetone is prepared by oxidation of isopropyl alcohol (2-propanol) with acidified potassium dichromate solution.
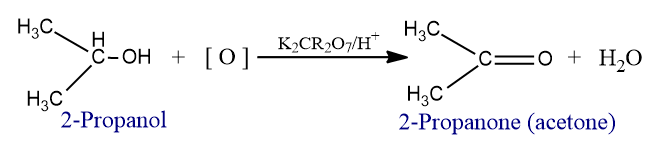
- It is prepared by the hydration of propyne in the presence of dilute sulphuric acid and mercuric sulphate.
- It may also be prepared by passing acetic acid vapors over alumina or manganous oxide at 400oC.
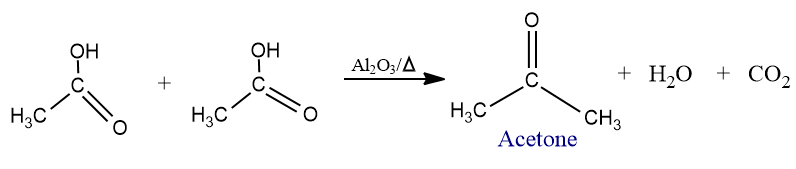
Laboratory Preparation of Acetone
Older method
Acetone is prepared in the laboratory by dry-distillation of anhydrous calcium acetate.
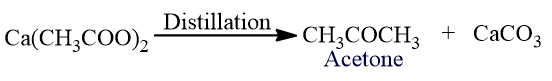
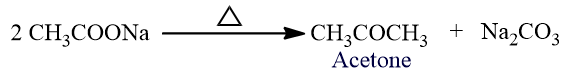
It is also obtained by the distillation of sodium acetate.
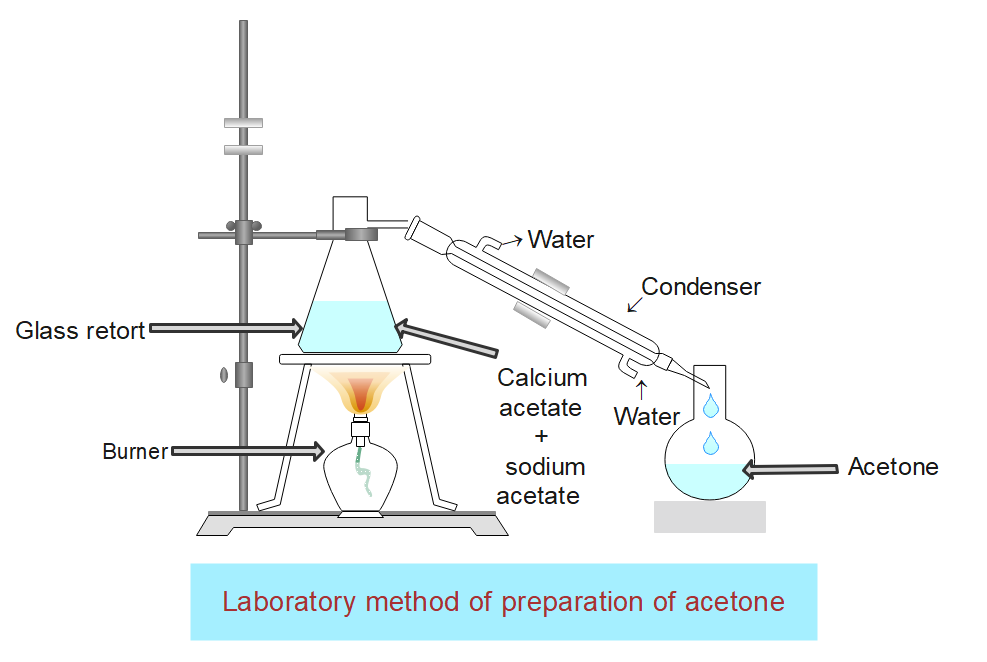
Crystalline calcium acetate is mixed with the same amount of sodium acetate and taken in the hard glass retort and it is heated. Vapors of acetone are evolved which are condensed to liquid acetone by passing through a condenser and the liquid acetone collects in the receiver.
The distillate is treated with a concentration sodium bisulphite solution and the white precipitate of the acetone-sodium bisulphite compound is filtered, washed with water, and is dried. The dry crystals of sodium bisulphite adduct are distilled with Na2CO3 solution when acetone is formed which is separated, dried with anhydrous CaCl2, and redistilled when pure acetone distills out.
New method
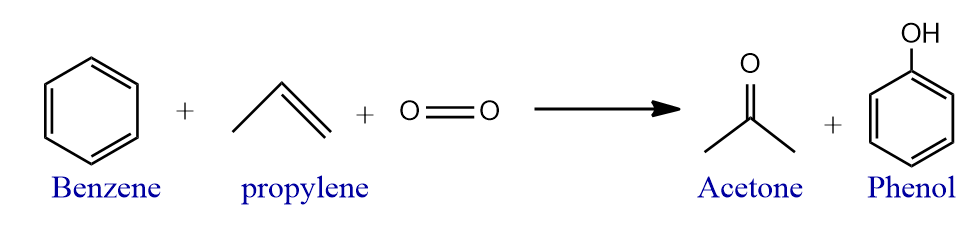
Nowadays acetone preparation method replaces by a more cost-effective and easy method. In this method propene is converted directly or indirectly producing acetone. The cumene process produces around 83% of acetone; as a result, acetone production is linked to phenol synthesis. In this process, Benzene is alkylated with propylene to yield cumene, which is then oxidized by air to give phenol and acetone.
Properties of Acetone
- It is a colorless, pleasant-smelling liquid.
- It is an inflammable liquid and boils at 58oC.
- It is miscible with water, alcohol, and ether in all proportions.
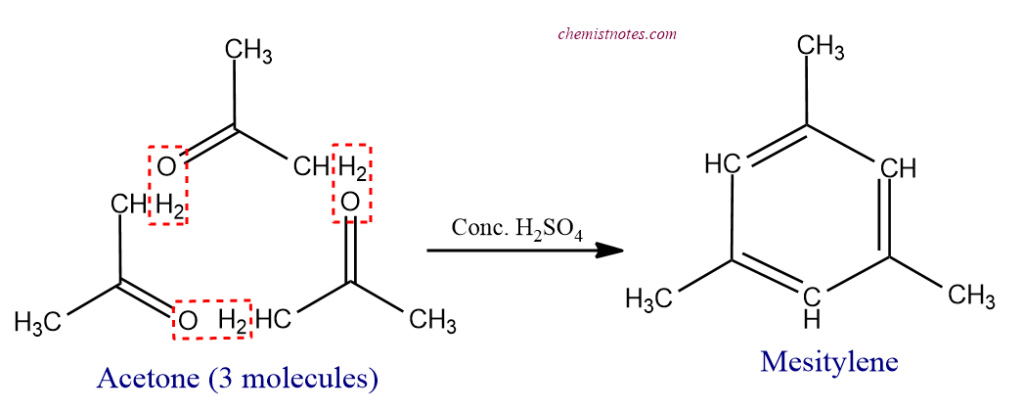
- When distilled with conc. H2SO4, acetone gives mesitylene, i.e., 1, 3, 5-trimethylbenzene.
Uses of Acetone
- It is used as a solvent for acetylene, cellulose acetate, cellulose nitrate, celluloid, lacquers, and varnishes.
- It is used to prepare a number of compounds such as chloroform, acetic anhydride, etc.
- It is also used in the laboratory as a polar, aprotic solvent in a variety of chemical reactions, including SN2 reactions.
- In academic laboratories, low-grade acetone is widely used as a glassware rinse solution to remove residue and solids prior to a final wash.
- It is used in the preparation of liquid nail polish and also used to remove nail polish from nails.
- It is also used for the extraction of essential oils.
- Dermatologists use acetone with alcohol for acne treatments and to exfoliate dry skin.
- Acetone is utilized in pharmaceutical companies as a solvent and as a denaturant in denatured alcohol. Some pharmaceutical medications contain acetone as an excipient.
Related video
FAQs
Is acetone in nail polish remover?
Acetone is used in the preparation of liquid nail polish.
What is acetone?
Acetone (or propanone or dimethyl ketone) is the simplest and most important aliphatic ketone with the chemical formula >C=O.
Is acetone is polar?
Acetone is used in the laboratory as a polar, aprotic solvent.