Table of Contents
ToggleLaboratory Preparation of ethoxyethane (diethyl ether), purification, and its uses in organic chemistry have been discussed here:
Principle involved in laboratory preparation of ethoxyethane
Ethoxyethane which is commonly known as diethyl ether is prepared in the lab by heating excess alcohol with concentrated H2SO4 at 140 degrees Celsius. This method of preparation is called Williamson’s continuous esterification.
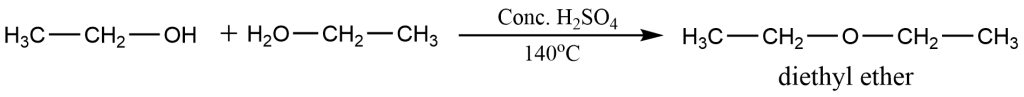
The reaction takes place in the following two steps:-
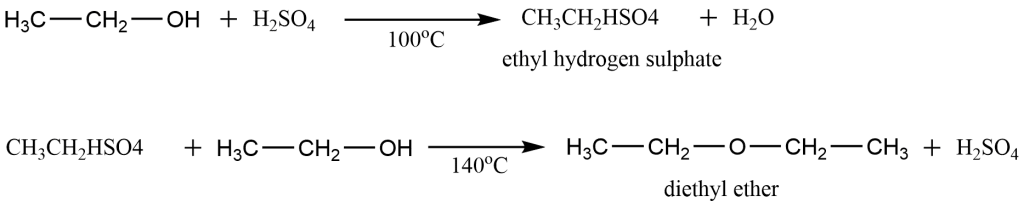
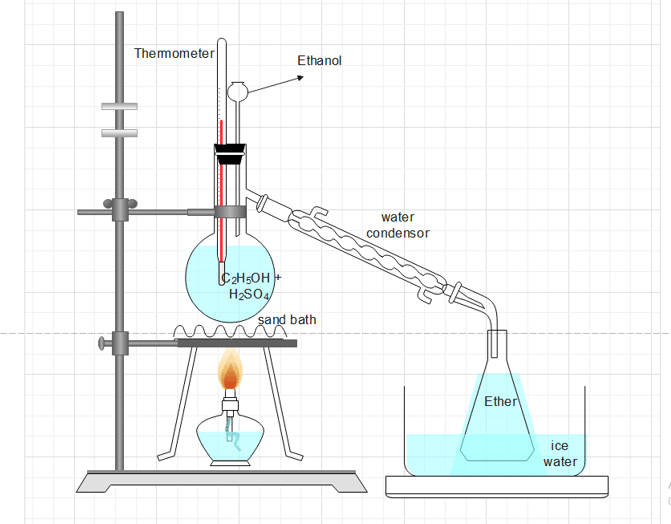
Procedure for the laboratory preparation of ethoxyethane (diethyl ether)
A 2:1 mixture of ethyl alcohol (100 mL) and concentrated H2SO4 (50 mL) is placed in a distillation flask fitted with a dropping funnel, thermometer, and water condenser. The flask is heated on a sand bath at 140oC. Ethanol is added at a rate that is approximately equal to the rate of distillation, ensuring that the ether generated is continually received in the receiver, which is kept cool in ice-cold water.
Purification of diethyl ether
Impurities such as sulphurous acid, ethyl alcohol, and water are present in the resulting ether. To purify the obtained ether, it is first treated with KOH or NaOH to remove the acid, and then with a 50% CaCl2 solution to remove the alcohol. After that, it is washed with water and dried over anhydrous calcium chloride. Finally, it is redistilled to produce pure, dry ether.
Uses of Diethyl Ether
- Used as a refrigerant
- Used as general anesthetic in surgery
- Used as a solvent in the preparation of Grignard reagent
- Used as solvents for oils, fats, gums, resins, plastics, etc.
Preparation of diethyl ether video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.






