Table of Contents
ToggleThe chemical reactions of phenols undergo various mechanisms due to the presence of the hydroxyl (-OH) group. Apart from reactions that directly affect the OH group, phenols undergo electrophilic aromatic substitution. This is because the ring of phenol is highly reactive toward electrophilic substitution. The phenoxide group (-O-), obtained by the ionization of phenol, has a full negative charge and hence releases electrons more strongly than the -OH group. Thus acidity plays an important part in ring substitution. Some of the important reactions of phenols are as follows.
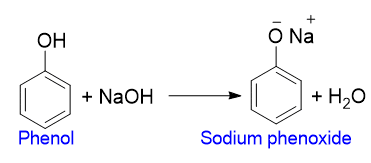
Salt formation of phenol
Phenols are weakly acidic in nature. Therefore, they react with alkali metals and alkalis to form their salts.
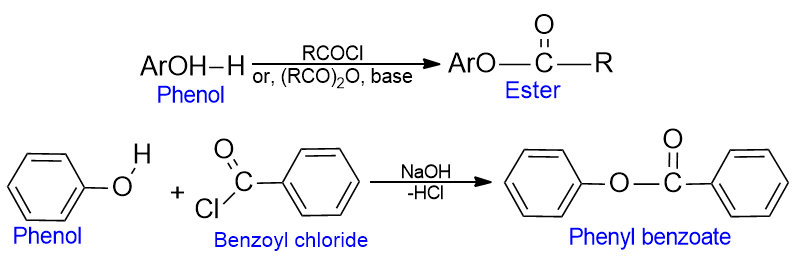
Ester formation of phenol
When phenols are warmed with acid chlorides or acid anhydrides, they form esters.
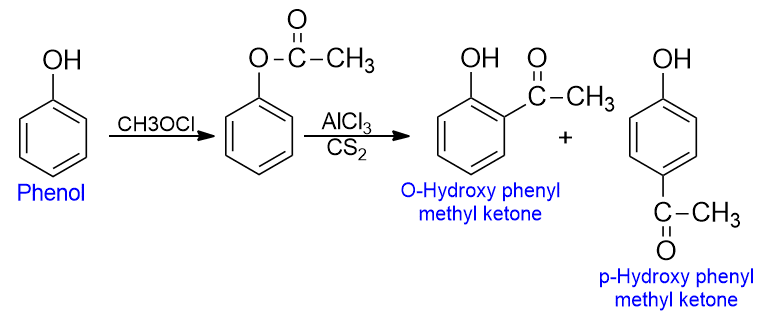
fries rearrangement
When phenols are heated with an acid chloride in the presence of anhydrous AlCl3, esters are formed which then undergo the rearrangement to give O- and P- acyl phenols (or phenolic ketones). Here the acyl group of ester migrates from the phenolic oxygen to an ortho or para position of the ring to form phenolic ketones. This reaction is called fries rearrangement.
Reimer-Tiemann reaction
When phenol is heated with chloroform and aqueous sodium hydroxide followed by hydrolysis, to aldehyde group (-CHO) gets introduced in the ring at a position ortho to the phenolic group. This reaction is called the Riemer-Tiemann reaction.
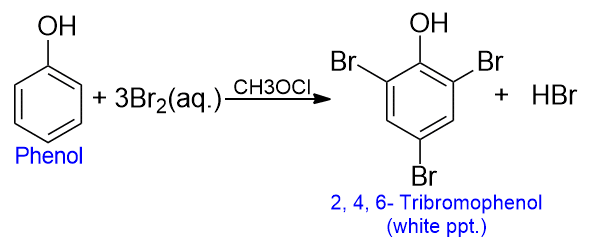
Bromination in phenol
The bromination of phenol is the most important chemical reaction of phenol. Phenol reacts with an aqueous solution of bromine to give a precipitate of 2, 4, 6 – tribromophenol, in which every hydrogen, ortho, or para, to the -OH group, is replaced. Certain other groups (deactivation groups such as -SO3H, -NO2, etc.) may also be displaced by bromine if they are present at O- or P- position to the -OH group.
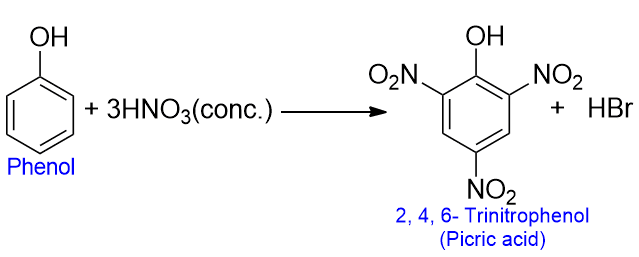
Nitration in phenol
Phenol gives 2, 4, 6 – trinitrophenol when treated with conc. HNO3. The yield is low because of the oxidation of the ring by conc. HNO3.
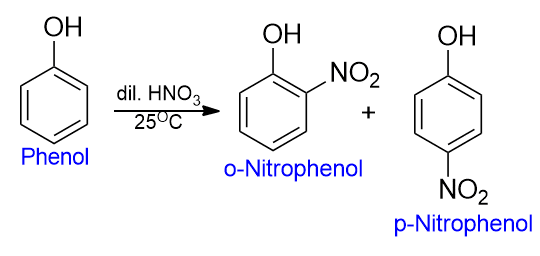
If the nitration is carried out by using dilute nitric acid at low temperatures, mono nitrophenols can be obtained.
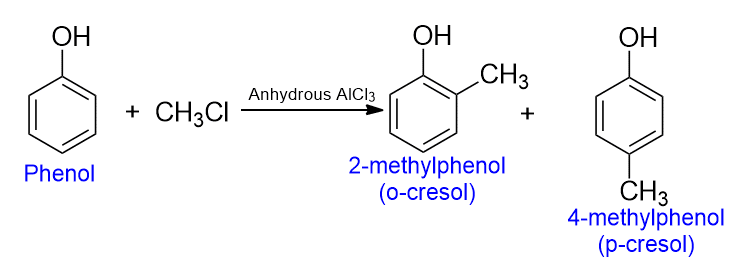
Friedel-crafts alkylation of phenol
Friedel-crafts alkylation is the important chemical reaction of phenol to form cresol. Phenol undergoes Friedel-Crafts alkylation to form alkyl phenols.
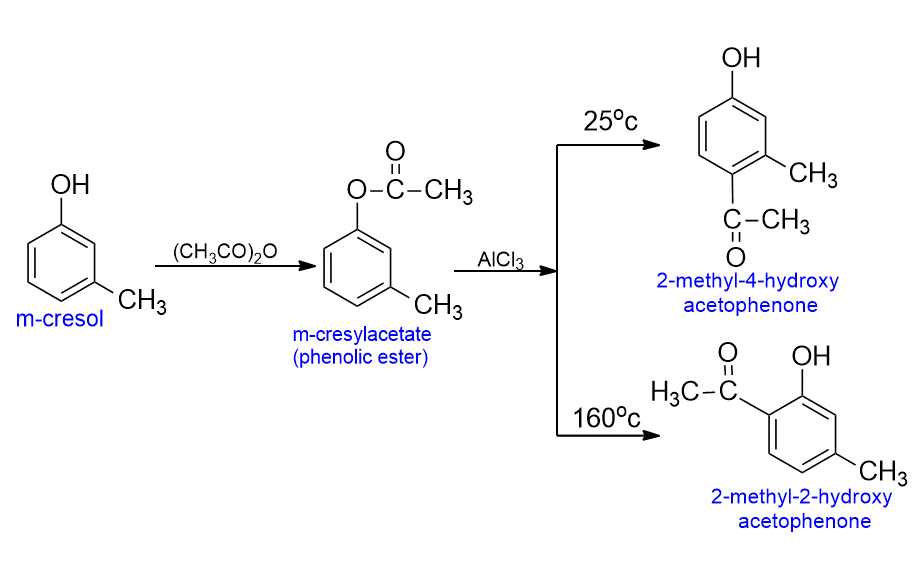
Friedel-crafts acylation of phenol
Phenolic ketones can be prepared by direct acylation of phenols, using acid chlorides or acid anhydrides. But they are most often prepared in two steps means of Fries rearrangement.
Nitrosation of phenol
When phenols are treated with nitrous acid at low temperatures, the nitrosonium ion (N+O) occupies the para-position to the -OH group. The reaction is called nitrosation.
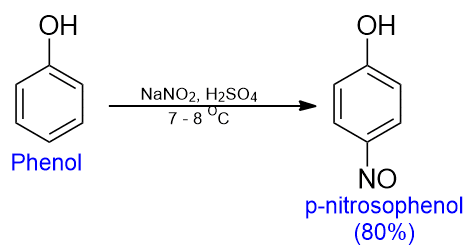
**Note: The -NO group is readily oxidized to the -NO2 group by HNO3. Thus this is the better way to synthesize p-nitrophenol than the direct nitration of phenol.
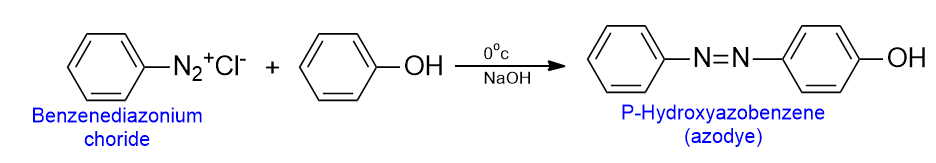
Coupling with the diazonium salt
In the alkaline medium, an ice-cold solution of phenol combines with the solution of diazonium salt to form an azo dye. The reaction is often called a diazo coupling.
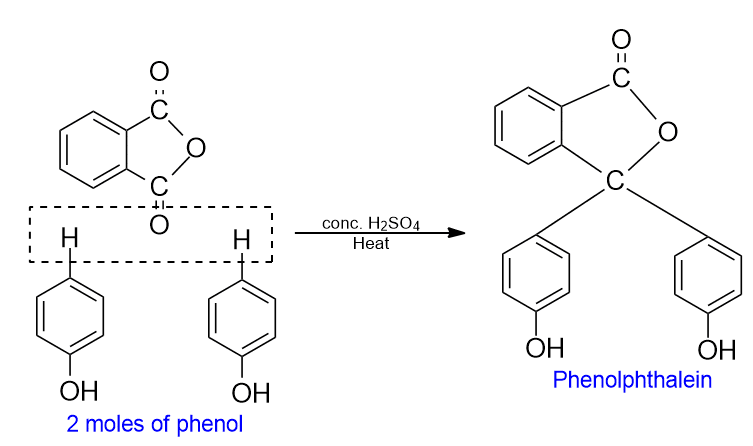
Reaction with phthalic anhydride
When phenol is treated with phthalic anhydride in the presence of conc. H2SO4, phenolphthalein is formed.
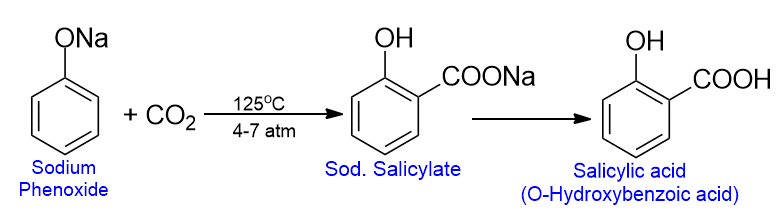
Kolbe synthesis
When sodium phenoxide is heated with carbon dioxide at about 125oC and under 4 to 7 atm pressure, sodium salicylate is formed as the major product. This product on acidification gives salicylic acid. This reaction involves the carbocation of phenolic acid and is called the Kolbe reaction.
Gattermann synthesis
The formylation (formyl, -CH=O) of certain aromatic compounds with hydrogen cyanide and hydrogen chloride in the presence of zinc chloride as the catalyst is called Gattermann synthesis. For example, when a phenol undergoes formylation, a -CHO group is introduced in the ortho position to -OH.
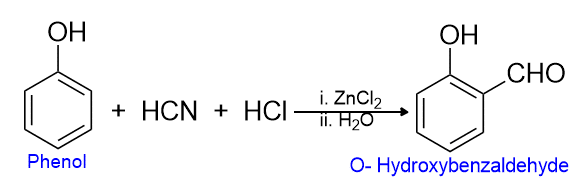
FAQs
What type of reaction is nitration of phenol?
The nitration of phenol is an electrophilic aromatic substitution reaction type.
What type of reaction occurs between phenol and bromine?
When bromine reacts with phenol electrophilic aromatic substitution reaction occurs between bromine and the aromatic ring of phenol.






