Table of Contents
ToggleFelkin Ahn model, Cram’s model, and Prelog generalization are some of the popular models developed to predict the stereochemical outcomes from the kinetically controlled addition of a nucleophile to the carbonyl group of the chiral aldehyde and ketone having a stereogenic center adjacent to the carbonyl group.
Brief introduction of Diastereoselective synthesis
In diastereoselective synthesis, a new chiral center is generally created on chiral substrate molecules. One of the most common methods of creating a new chiral center is the addition of groups to the diastereotopic faces of double bonds. For example, nucleophiles can attack from both Pie faces of the C=O group of carbonyl compounds under kinetic controls, thus diastereomers are formed.
To determine which diastereomer is formed as a major product i.e selectivity in the addition of nucleophiles to the double bonds especially the carbonyl group, above mentioned models have been developed.
Felkin Ahn model
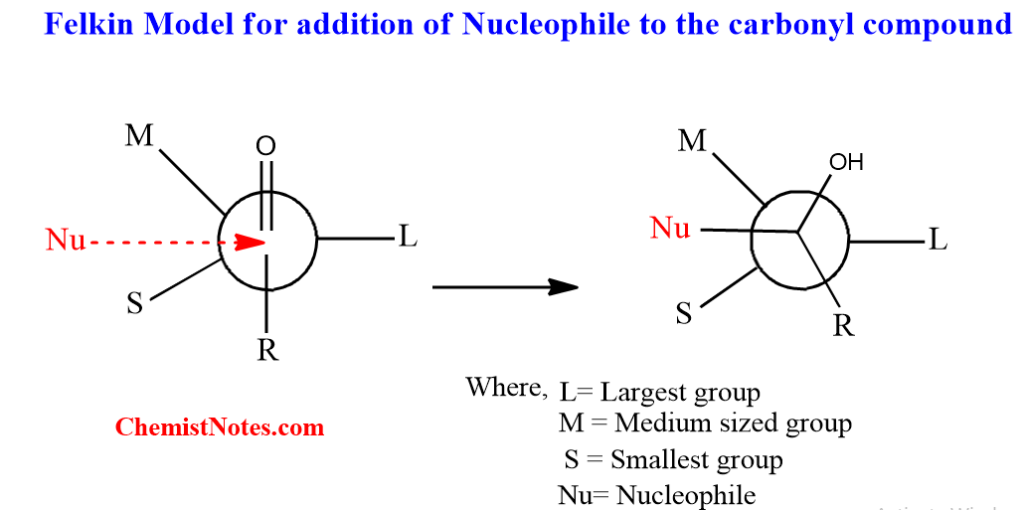
One of the most accepted models to predict the stereochemical outcomes in these reactions is the Felkin model, also known as the Felkin Ahn model.
In the Felkin Ahn model, the largest or bulkiest of the alpha ligands(L) prefers a conformation where it is perpendicular to the plane of the carbonyl C=O group and anti to the incoming nucleophile. The medium-sized group(M) is placed gauche to the carbonyl C=O. Thus, in this conformation, the non-bonded interaction between the nucleophile and small ligand(S) group is minimized. This conformation led to the preferred transition state.
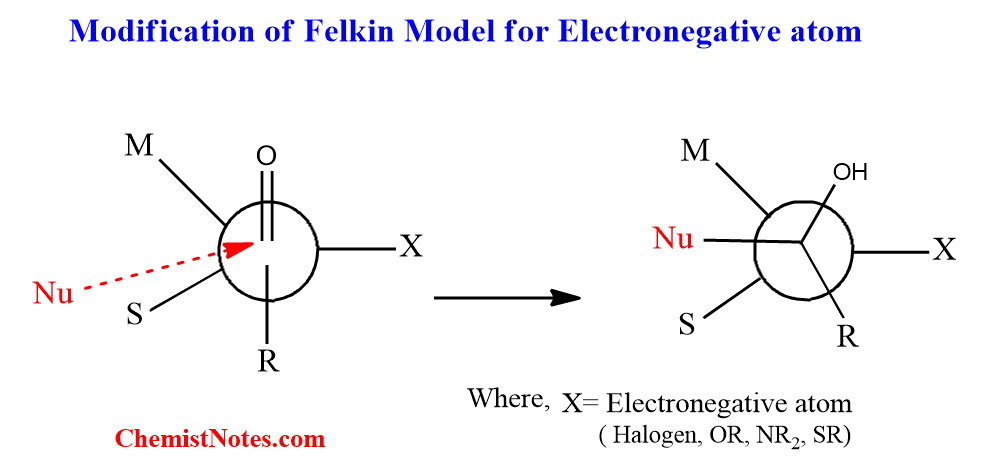
Effect of Electronegativity in Felkin Model
There is a slight modification in the confirmation of the Felkin model if an electronegativity atom and group such as halogens, OR, NR2, or SR is present at the α-position to the carbonyl group. The slight modification is that in the Felkin-Ahn model, this electronegative atom or group becomes equivalent to the largest group. The coulombic repulsion between the electronegative atom and the approaching nucleophile is reduced by this orientation of the electronegative atom.
Effect of chelation in Felkin model
Because certain heteroatom-based functional groups such as OR, NR2, and SR are Lewis bases metals, the α-heteroatom substituted situation can be more complicated. As a result, the carbonyl C=O and X-group will interact in certain organometallic systems to chelate the metals, which substantially alters the routes for addition.
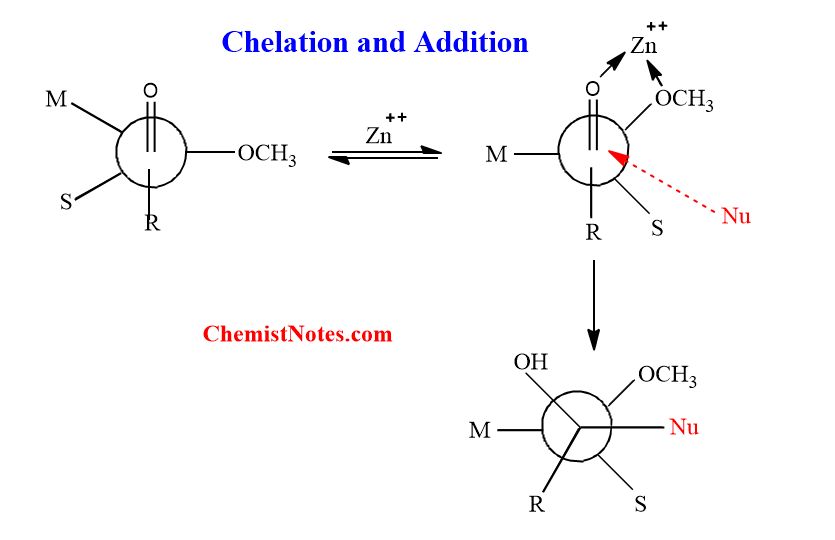
When metal is Li+(typically), Na+, or K+, the Felkin Ahn model is used because such chelate doesn’t exist. However, such chelation happens when the metals are Mg++(typically), Zn++, Cu++, Mn++, and so on.
Due to chelation, the -X group is forced to be held close to the carbonyl C=O. When nucleophile attacks, it takes the Burgi-Dunitz path.
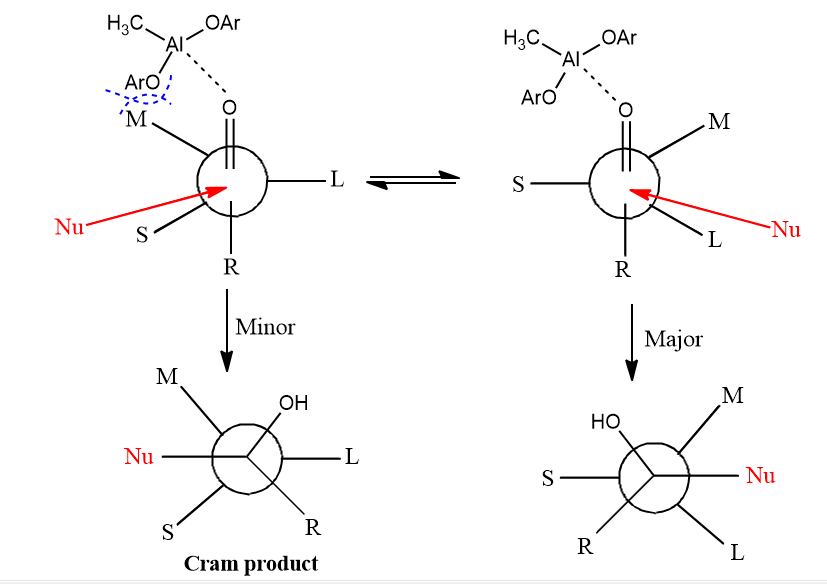
Effect of complexation of bulky Lewis acid in Felkin model
When there is bulky Lewis acid, it combines with the carbonyl group’s oxygen. Because of its bulky size, it displays steric interaction with the medium-sized group making the conformation unstable. However, there occurs conformation interconversion in which there is no or little steric interaction with the small-sized group. This conformation gives a product as major.
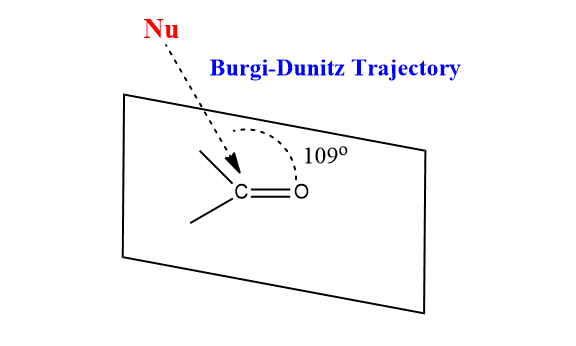
Burgi-Dunitz Trajectory/Model
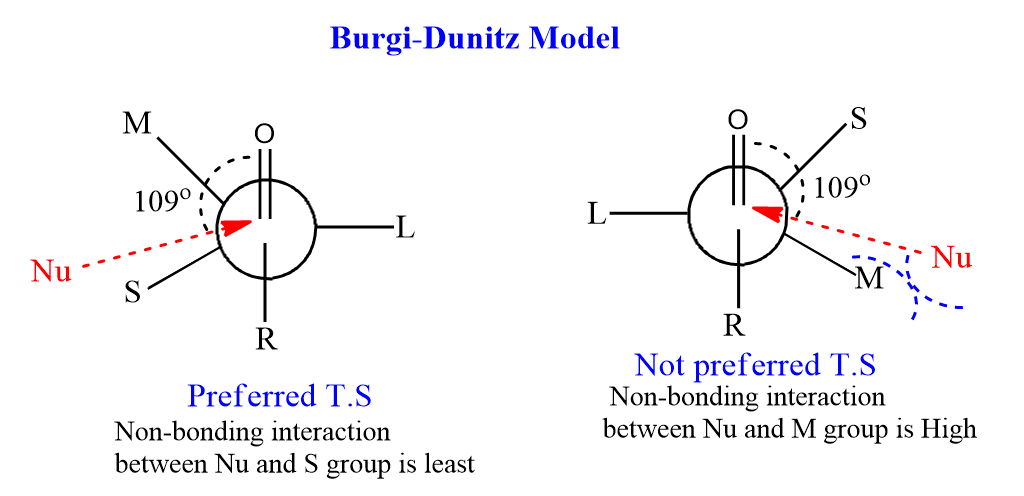
According to the Burgi-Dunitz model, the nucleophile prefers to attack carbonyls, not at a bond angle of 90o to the C=O, but at an angle of 1090 with respect to the C=O. This specific angle of approach by nucleophiles during its attack on the carbonyl carbon is known as the Burgi-Dunitz trajectory.
In this case, it is not necessary to assume the medium-sized group should be gauche to the oxygen of C=O in the Transition state.