Table of Contents
ToggleBenzene is the simplest and most important compound of aromatic hydrocarbons. It was first discovered by Michael faraday in 1825. It was given the name benzene because it was first prepared from benzoic acid isolated from ‘gum benzoin’. In 1845 Hoffmann isolated it from coal tar.
Benzene Ring
The benzene ring is a resonance hybrid of two Kekule formulae and is not identical with either of the two structures.
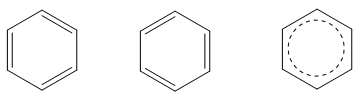
Structure of Benzene
The following facts were known before the structure of benzene was proposed:
- Benzene has the molecular formula C6H6.
- Benzene undergoes substitution reactions rather than addition reactions.
- Benzene forms only one kind of monosubstituted product.
- Benzene forms only three kinds of disubstituted products distinguished as ortho-,meta- and para-substitution products.
- Benzene is stable towards oxidizing agents like KMnO4 under usual conditio and hence, is highly stable.
Kekule Rules of Benzene
The German chemist August Kekule (1865) suggested that the benzene molecule is made up of a hexagon of six carbon atoms joined by alternate single and double bonds, and with a hydrogen atom attached to each carbon atom. The cyclic structure of benzene containing these alternate double and single bonds is known as Kekule’s formula.
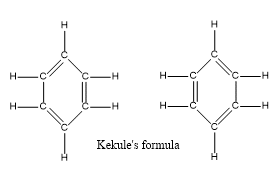
Resonance structure of Benzene
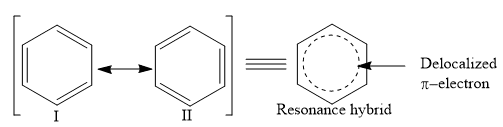
According to resonance theory, benzene is a resonance hybrid of two Kekule formulae I and II and is not identical with either of the two structures.
These two structures are identical and differ only in the arrangement of pi-electrons. Since these two structures are exactly equivalent and hence, of exactly the same stability. They make an equal contribution to the resonance hybrid, therefore, the stabilization is due to the agreement, and it is high resonance energy that is responsible for ‘aromatic properties’.
Resonance structure of Benzene
Resonance structure also shows that carbon-carbon bonds in benzene are neither single bonds nor double bonds but something halfway in between. This explains why benzene gives substitution reactions, while it undergoes addition reactions under forcing conditions. As all the carbon-carbon bonds in the resonance hybrid of benzene are equivalent, 1,2- and 1,6- positions are obviously equivalent.
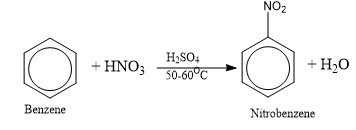
Nitration of Benzene
It is carried out by heating benzene with a mixture of concentrated nitric acid and sulphuric acid (nitrating mixture) to 50 – 60o C.
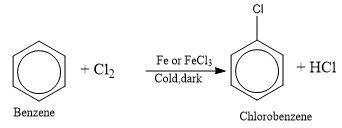
Halogenation of Benzene
Benzene reacts with halogen (chlorine or bromine) in the cold, dark, and in the presence of a halogen catalyst) like AlCl3, FeCl3, or FeBr3 to form halobenzene. FeCl3 or FeBr3 can be made by a direct combination of the elements. Therefore, it is sufficient to add iron filings to the reaction mixture which are converted by the halogen into the corresponding ferric halide catalyst.
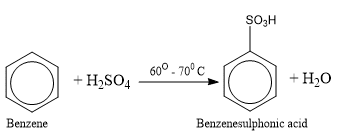
Sulphonation of Benzene
Benzene on heating with fuming sulphuric acid (concentrated sulphuric acid containing some dissolved SO3) undergoes sulphonation forming benzene sulphonic acid.
Physical properties
- Benzene is a colourless liquid with a characteristic smell.
- Its boiling point is 80° C and its freezing point is 5.5° C.
- It is insoluble in water but is soluble in alcohol and ether in all properties.
- Its vapour is toxic and carcinogenic.
- It acts as a very good solvent for fats, resins, sulphur, iodine, and a large number of other organic compounds.
Benzene in sunscreen
It’s important to note that benzene is not an ingredient in sunscreen because the benzene chemical is carcinogenic.
FAQs
What is benzene?
Benzene is the simplest and most important of the aromatic hydrocarbons.
Benzene in sunscreen
It’s important to note that benzene is not an ingredient in sunscreen because the benzene chemical is carcinogenic.






