Table of Contents
ToggleAvogadro’s Hypothesis, introduced by Amaedo Avogardro in 1811, explains that in every gas the number of molecules is proportional to the volume. He replaced the term ‘atoms’ in the Berzelius hypothesis with the term ‘molecules’ and postulated a law, popularly known as Avogadro’s hypothesis.
Avogadro’s hypothesis states, “Equal volumes of all gases and vapors contain an equal number of molecules under similar conditions of temperature and pressure.” According to this hypothesis, under the same temperature and pressure, the volume of a gas depends upon the number of molecules or moles but not upon their size or mass. Under the assumption of an ideal (perfect) gas, this empirical relationship can be derived from the kinetic theory of gases.
This law is valid for real gases at low pressure and high temperature.
Example of Avogadro’s law
If we consider 2 liters of nitrogen, carbon dioxide, and hydrogen each under STP, they will contain an equal number of molecules however their mass is different. This hypothesis is experimentally verified and is called Avogadro’s law. Avogadro’s law can explain gaseous reactions without violating Dalton’s atomic theory.

Let 1 volume of gas contain ‘n’ molecule then apply Avogadro’s law

The above experimental result shows that 1 molecule of hydrogen chloride is produced from 1/2 molecule of hydrogen and half molecule of chlorine. The fraction of molecules is possible because a molecule contains more than one atom. Hence Avogadro’s hypothesis is in accordance with dalton’s atomic theory and Gay-Lussac’s law of gaseous volume.
Application of Avogadro’s law
Avogadro’s law has numerous applications, some of which are as follows:
1. Determination of atomicity of elementary gases
Atomicity is the number of atoms present in one molecule of an element or compound. elementary gases are composed of atoms of the same element. For example, H2, Cl2, O2, N2, etc. Other types of gases are compound gases like CO2, NH3, SO2, N2O5, etc. Avogadro’s law is applied to determine the atomicity of elementary gases. Elementary gases are diatomic, i.e., one molecule contains two atoms. For Further illustration, we can take the example of oxygen.
1.1.Oxygen is Diatomic gas
Oxygen reacts with hydrogen to give water vapor. Experimentally, it has been found that hydrogen and oxygen react in the ratio of 2:1 by volume to give 2 volumes of water vapor under similar conditions of temperature and pressure. This experimental result is formulated as follows:
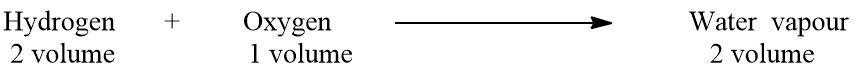
Let one volume of gas contain ‘n’ molecules, then by applying Avogadro’s hypothesis,
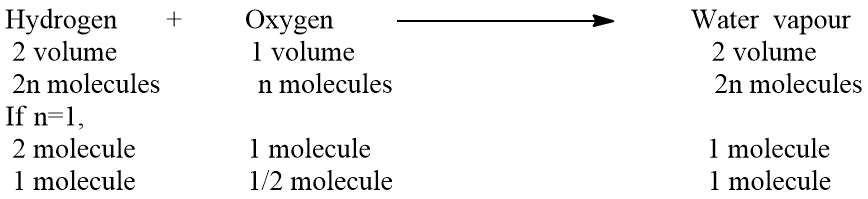
One molecule of water vapor contains one molecule of hydrogen and 1/2 molecule of oxygen. From Avogadro’s law, it has been proved that one molecule of hydrogen contains 2 atoms. The valency of hydrogen is one. Hence, two atoms of hydrogen can satisfy the valency of only one atom of oxygen because the valency of oxygen is two. From the above discussion, we come to know that one molecule of water vapor contains only one atom of oxygen. This one atom is derived from its half molecule. Hence, we can write,
1/2 molecule of oxygen contains 1 atom or 1 atom of oxygen contains 2 atoms.
Hence, by applying Avogadro’s hypothesis we are able to determine the atomicity of oxygen i.e, 2.
2. Deduction of the relationship between molecular mass and vapor density
Molecular mass or molecular weight is the mass of one molecule of a substance as compared to the mass of one atom of hydrogen.
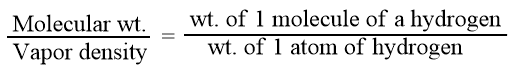
From Avogadro’s law, we know that hydrogen is diatomic i.e. a molecule containing two atoms. Hence, we can write
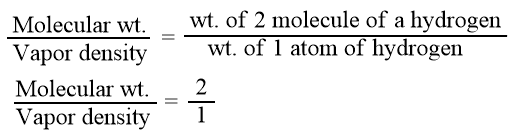
Mol. wt. = 2 × V.D.
Hence, the molecular weight of a gaseous substance is twice its vapor density.
3. Derivation of the relationship between gram molecular mass and volume of gas
The molecular weight expressed in grams is called gram molecular mass. Experimentally, it has been found that the gram molecular mass of any gas or vapor at STP occupies 22.4 liters. This experimental observation is theoretically explained by applying Avogadro’s law.
4. Derivation of the relationship between gram molecular weight and number of molecules
From Avogadro’s law, we know that the gram molecular weight of any gas or vapor occupies 22.4 liters at STP. This quantity is also called molar volume i.e, the volume occupied by one molecule. According to Avogadro’s law, we know that the equal volume of all gases and vapors contains an equal number of molecules under similar conditions of temperature and pressure.
Since one mole of any gas or vapor at STP occupies 22.4 liters. Due to equal volume, it must contain the same number of molecules. Experimentally it has been found that 22.4 liters of gas at STP contain 6.023 × 1023 molecules.
5. Determination of molecular formula from volume composition of a gas
Avogadro’s law is also applied to determine the molecular formula of a gaseous compound from its volume composition and molecular mass. It can be explained by considering an oxide of nitrogen having a molecular mass of 60 and one volume of which is composed of half of the volume of nitrogen and half of the volume of oxygen i.e. volume ratio of nitrogen: oxygen: nitrogen oxides is 1:1:2 under the similar condition of temperature and pressure.
FAQs
What is Avogadro’s hypothesis?
Avogadro’s hypothesis states, “Equal volumes of all gases and vapors contain an equal number of molecules under similar conditions of temperature and pressure.”
What evidence supports Avogadro’s hypothesis?
The fraction of molecules is possible because a molecule contains more than one atom.






