Table of Contents
ToggleKinetic molecular theory of gases is an theoretical model which was put forwarded by Bernoulli in 1738 and later it was further developed by Kronig, Clausius, Maxwell, Boltzmann and other scientists.
Other gas laws such as Boyle’s law, Charle’s law, Avogardo’s hypothesis, Graham’s law of diffusion etc were totally based on experimental observations and there was no any theoretical background to explain the observed behavior of gases. Thus, in order to explain the observed behavior of a gases kinetic theory of gases was proposed.
Postulates of kinetic molecular theory of gases
The main postulates or assumptions of kinetic theory of gases are as follows:
- All gases consists of very large number of minute particles called molecules. The gas molecules are so simple that their actual volume is negligible as compared to the total volume occupied by the gas.
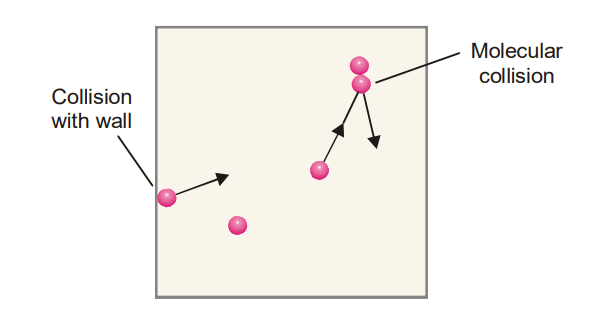
- The gas molecules are in state of random motion in all possible direction and collides with one another and with the wall of the container.
- The collisions between the molecules are perfectly elastic. It means, there is no loss of energy when gas molecules colloid with one another or against the walls of the container.
- The distance between the gas molecules being very large, there is no effective force of attraction between molecules and the walls of the container. They were completely independent of one another and laws of motion can be applied to them.
- The pressure exerted by a gas is due to the continuous bombardment of the moving molecules on the walls of the containers.
- The average of kinetic energy of the molecules is directly proportional to the absolute temperature of the gas.
- There is no effect of gravity on the motion of the gas molecules.
Kinetic theory of gases equation
On the basis of postulates of kinetic molecular theory of gases, an equation can be derived known as kinetic gas equation for gases. The expression of kinetic gas equation is given as follows:
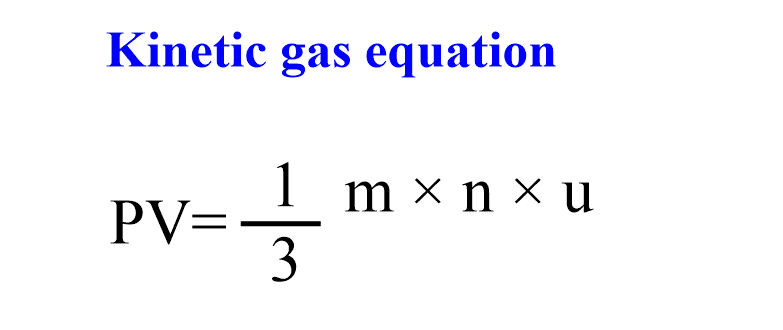
where, P= pressure exerted by the gases,
V= volume of gas,
m=mass of each gas molecules,
n=total number of gas molecules,
u=root mean square velocity of gas molecules.






