Table of Contents
ToggleGas laws describe the general behavior of gases. When conditions are normal, all gases exhibit similar behavior. However, even minor changes in physical conditions such as pressure, temperature, or volume cause a deviation. Thus, gas laws define relationships among the temperature, pressure, and volume of a gas. The gas laws are:
- Boyle’s law
- Charle’s law
- Gay-Lussac’s law
- Avogadro’s law
- Combined gas law
Boyle’s Law
Boyle’s law, given by Robert Boyle in 1662, describes the pressure-volume relationship of gases at a constant temperature. Boyle’s law states that “At a constant temperature, the volume of a given mass of a gas is inversely proportional to its pressure“.
V ∝ 1/P (At a constant temperature for a given mass of gas)
V = k × 1/P, where V is the volume, P is the pressure and k is the proportionality constant
PV = k = a constant
Thus, Boyle’s law can be also stated as “the product of the pressure and volume of the given mass of gas is constant at a constant temperature“.
If P1, V1 are the initial pressure and volume of a given sample of gas at temperature T K and P2, V2 the changed pressure and volume at the same temperature, then
P1 V1 = P2 V2
Thus, the volume of a gas corresponding to any changed pressure can be calculated at a constant temperature using the above Boyle’s law equation.
Charle’s Law
Charle’s law, given by Jacques Charles in 1787, describes the relationship between volume and temperature of gases at a constant pressure. Charle’s law states that “At constant pressure, the volume of the given mass of a gas is directly proportional to the absolute temperature of the gas“. This law can be expressed mathematically as:
V ∝ T (At a constant pressure for a given mass of gas)
V = k × T, where V is the volume, T is the pressure, and k is the proportionality constant

Thus, the volume of a gas corresponding to any changed temperature can be calculated at a constant pressure using the above Charle’s law equation.
Gay-Lussac’s law
Gay-Lussac’s law, given by Joseph Gay Lussac in 1802, describes a general relation between
the temperature and pressure of a gas at constant volume. Gay-Lussac’s law states that “At constant volume, the pressure of a given mass of gas is directly proportional to the absolute temperature of the gas“.
P ∝ T (At a constant volume for a given mass of gas)
P = k × T, where P is the pressure, T is temperature, and k is the proportionality constant
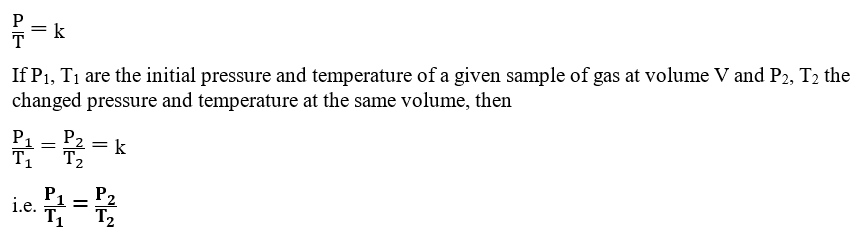
Thus, the pressure of a gas corresponding to any changed temperature can be calculated at a constant volume using the above Gay-Lussac’s law equation.
Avogadro’s Law
Avogadro’s law, given by Amadeo Avogadro in 1811, describes the relationship between volume and the number of molecules of gas at constant temperature and pressure. Avogadro’s law states that “Under similar conditions of temperature and pressure, equal volumes of all gases contain an equal number of molecules“.
V ∝ n, (At constant temperature and pressure)
Number of moles, n = Number of molecules / 6.023 × 1023
or, n = n, / N
or, n ∝ n,
V ∝ n i.e. volumes of gases at constant temperature and pressure are directly proportional to their number of moles.
V = k × n, where V is the volume of gas, n is the number of moles, and k is the proportionality constant
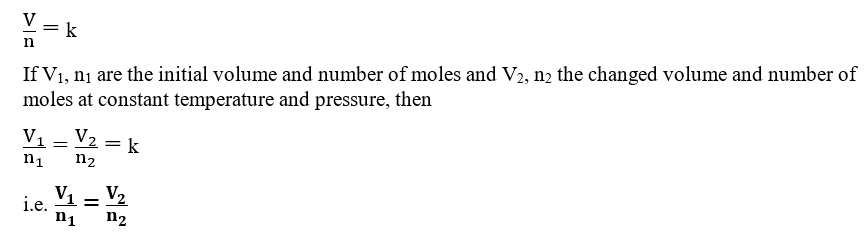
Combined gas law
Combined gas, the combination of Boyle’s law and Charles’ law describes the effect of changes in pressure and temperature on the volume of a gas. The combined law states that “For a given mass of gas, the volume is directly proportional to the absolute temperature while inversely proportional to the pressure.
From Boyle’s law, V ∝ 1/P
From Charle’s law, V ∝ T
On combining both,
V ∝ T/P
V = k × T/P

This equation is the required combined gas law equation.
Gas laws Video
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry. Holt, Rinehart, and Winston.
- J. N. Gurtu and A. Gurtu, Advanced Physical Chemistry Experiments, (6th Edition).






