Table of Contents
ToggleJohn Dalton, an English chemist proposed Dalton’s atomic theory by adopting an ancient theory concerning the ultimate constitution of matter which had been expounded by the early Hindu and Greek philosophers. John Dalton published a book in 1808 A.D., “The theory of chemical reactivity” in which he explained the existence of atoms in a scientific way. Dalton developed it into the modern atomic theory to explain the law of chemical combination.
Postulates of Dalton’s Atomic Theory
- Atoms are the extremely tiny, indivisible, and invisible basic building block of all matter.
- Atoms of an element are similar in all respect while atoms of different elements are different in terms of properties and weight.
- Atoms cannot be destroyed or generated, which means that atoms of one element cannot be transformed into atoms of another. This is also known as the law of matter’s indestructibility.
- Simple whole-number ratios can be used to combine atoms to create a compound. During combination, the atoms of one element are not changed into those of another element.
The advancement of modern sciences has been significantly influenced by Dalton’s atomic theory. However, this idea is currently being changed for a lot of reasons. According to Dalton’s theory of the atom, an element’s atom is its tiniest particle. But, nowadays we know that atom is not the smallest particle of an element. It has been found that an atom contains approximately forty subatomic particles. Similarly, atoms of an element may be different in terms of mass number as well as physical properties. We know an element may have different types of isotopes. However, atoms of an element have identical chemical properties. The atoms of different elements may have the same mass number i.e. isobars.
Nowadays atoms of one of the elements can be changed into the atoms of another element by nuclear reactions. For example, a nitrogen atom can be changed into an oxygen atom by bombarding by alpha-particles i.e.
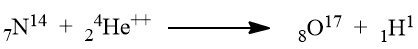
Atoms can combine between themselves to give molecules. The combining ratio may be simple or complex however, it is an integral ratio. The above explanation shows that Dalton’s atomic theory is no more applicable in its original form.
Limitations of Dalton’s Atomic Theory
The advancement of knowledge in the composition of matters gradually posed some limitations to Dalton’s atomic theory. Some of the considerable drawbacks of this theory are mentioned below:
1. Atoms are divisible
The smallest unit of matter involved in chemical processes is the atom, however, even atoms are made up of other tiny particles like electrons, protons, neutrons, positrons, neutrinos, mesons, quarks, etc. Although many subatomic particles have been identified, electrons, protons, and neutrons remain the fundamental particles.
2. Elements possess isotopes
All of the atoms of the same element should have equal physical and chemical characteristics. However, this idea is false because isotopes exist for many different elements. Isotopes are distinct atoms with the same atomic number but different atomic masses within the same element. The physical properties of the isotopes are quite different and the chemical properties remain similar. For example, hydrogen has got three isotopes, i.e. protium, deuterium, and tritium, 1H1, 1H2, and 1H3 respectively. Similarly, carbon exists in three isotopic forms:6C12, 6C13, and 6C14.
3. Molecules are the smallest units
A molecule can also be considered as the smallest particle with independent existence which takes part as a unit in chemical reactions like the atoms do. The molecules may be homoatomic or heteroatomic in their composition. The unitary behavior of some molecules makes dalton’s atomic theory defective. For example, H2, N2, O2, etc. are homoatomic molecules, and CO, NO, CaO, etc are heteroatomic molecules.
4. Creation and destruction of atoms
There are about 120 elements discovered yet. Many of them are natural and some others are made artificially by human beings. One atom can be changed into another through radio activities. Emission of radiations like α, β, ϒ, etc, particles from some of the heavy elements generates new elements. For example, Uranium radiates alpha particles to produce thorium and radium.
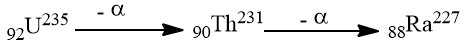
Some elements have been synthesized in the laboratory by artificial means. For example, Curium, Einsteinium, Mendelevium, Nobelium, etc. This type of natural and artificial interconversion of the elements is not considered by Dalton in his atomic theory.
Dalton’s atomic theory and the law of conservation of mass
According to Dalton’s atomic theory, the atom is neither created nor destroyed. So, during the reaction, the total number of atoms on the product side is equal to the total number of atoms on the product side. Due to the same number of atoms, the total mass of all the atoms on the reactant side is equal to the total mass of all the atoms on the product side i.e. mass remains conserved during the reaction. Thus Dalton’s theory explains the law of conservation of mass.






