Table of Contents
ToggleThe ideal gas law, also known as the ideal gas equation, applies to all gases that show ideal behavior, or that perfectly obey the gas laws. Gases are considered ideal only if (i) the volume of the molecules is negligibly small in comparison to the volume of the gas, (ii) a large number of molecules that constitutes the gas move randomly and obey Newton’s laws of motion, (iii)collisions are perfectly elastic, and (iv) no intermolecular forces with other gas particles.
Ideal gas law proposed by Benoît Paul Émile Clapeyron in 1834 describes the relationship of the product of pressure and volume of gas with the product of Universal gas constant, number of moles of gas, and temperature. Ideal gas law states that. ” The volume of a given amount of gas is directly proportional to the number of moles of gas and absolute temperature, while inversely proportional to the pressure“. At low pressures, the ideal gas equation is applicable for all gases.
The Ideal Gas Equation is given by:
PV = nRT
where P = Pressure, V = volume, n= amount of substance, T= temperature, R= Universal gas constant
Derivation of Ideal gas equation
From Boyle’s law: V ∝ 1/P (At a constant temperature for a given mass of gas)
From Charle’s law: V ∝ T (At a constant pressure for a given mass of gas)
From Avogadro’s law: V ∝ n (At constant temperature and pressure)
By combining all these three laws, we get
V ∝ nT/P
V = R × nT/P, where R is the proportionality constant called universal gas constant
PV = nRT……………………(i)
This equation (i) is called an ideal gas equation.
For 1 mole of gas, PV = RT
Units of Universal gas constant (R)
In the gas law equation, PV = nRT, R represents work done per degree per mole. Thus, it is expressed as ergs K-1mole -1 in CGS units and Joule K-1mole -1 in SI units.
Numerical Value of R
The numerical value of R is determined by the units used to express pressure and volume.
When pressure is expressed in (P) = 1 atmosphere, n = 1, and T = 273.15K, V = 22.414 litres, then
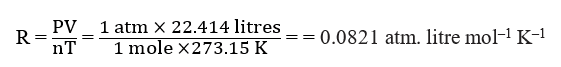
When pressure is expressed in dynes/ cm2 and volume in a cm3, then
P = 1 atm. = 76 cm length of mercury column = 76 × 13.59 × 981 dynes/cm2
V = 22.414 litres = 22414 cm3 , thus
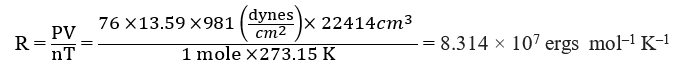
As 1.0 × 107 ergs = 1 Joule, the value of R in the SI unit is, R = 8.314 Joule K-1 mol-1
Since, 1 calorie = 4.184 Joule, R = 1.987 cal. K-1 mol-1
Ideal gas equation video
FAQs/MCQs
what is r in the ideal gas law equation apex?
R in the ideal gas law equation is a constant known as the universal gas constant.
which equation agrees with the ideal gas law?
PV = nRT
write the ideal gas equation.
PV = nRT where P = Pressure, V = volume, n= amount of substance, T= temperature, R= Universal gas constant
References
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry. Holt, Rinehart, and Winston.






