Table of Contents
ToggleNuclear reactions is a process that proceeds with a change in the nucleus composition to form an atom of a different element. In general, when two atomic nuclei or the nucleus of an atom and a proton, neutron, or electron from outside the atom collide, new particles are produced from the original particles. The conversion of one element to another by a nuclear transformation/change is called transmutation.
A nuclear reaction can be represented by an equation similar to that used in a chemical reaction.
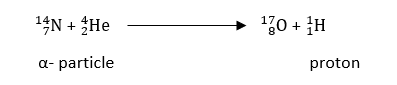
The target nucleus is the one in which the bombardment occurs. As a result of nuclear fusion, the nucleus is produced. A small particle released is referred to as an ejectile, while a projectile used for bombardment is known as a bombarding particle.
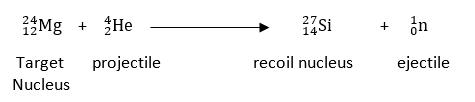
Balancing nuclear reactions
A nuclear reaction should also be balanced similar to a chemical reaction; charge and mass number should be the same on the two sides of the equation.
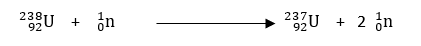
Total mass of reactant (238 + 1 = 239) = Total mass of product (237+2 = 239)
Total charge of reactant (92+0 = 92) = Total charge of product (92+0 = 92)
Theory of Nuclear reactions
The Bohr theory can be used to describe the mechanism of nuclear reactions. This theory states that the target nucleus is combined with projectile particles to form an unstable intermediate compound nucleus. Due to its high energy, it is unstable and hence undergoes fragmentation giving the product or recoil nucleus. The quantity of energy associated with the intermediate, however, governs the type of reaction.
Types of Nuclear reactions
Nuclear reactions are of two types:
- Nuclear reactions based on overall energy transformation
- Nuclear reaction based on nature of bombarding particle
A. Nuclear reactions based on overall energy transformation
It is further categorized into:
1. Nuclear fission reactions
Nuclear reaction in which a heavy nucleus splits into two or more lighter nuclei of nearly equal size is called nuclear fission reaction.
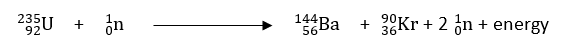
This is further of two types; controlled nuclear fission; and uncontrolled nuclear fission
- Controlled nuclear fission: In this reaction, moderators like graphite or heavy water are combined with fissionable material to slow down the neutron’s speed. These cause the neutron to slow down and collide with the nucleus, which triggers further controlled fission.
- Uncontrolled nuclear fission: In this instance, neutrons released from the reaction strike nuclei again to trigger further fission until all of the uranium has been consumed. This theory is used to create atom bombs since it produces enormous amounts of energy.
2. Nuclear fusion reaction
Nuclear reaction in which two lighter nuclei combine with each other to give a stable nucleus with higher mass is a nuclear fusion reaction. A large amount of energy is liberated in this process.
For example, energy in sun and stars are produced by the nuclear fusion reaction.
3. Spallation reaction
The word spallation, derived from ‘to spall’ (the verb) means to break up by splitting smaller pieces from the larger ones. In this reaction, a high-velocity projectile with an energy greater than 400 MeV is absorbed by a heavy nucleus to create a new nucleus that contains a significant amount of light particles. The atomic number of the nucleus created by the spallation reaction is between 10 and 20 units lower than the nucleus’s original number.

4. Projectile Capture reaction
In this reaction, the bombarding particle is captured by the target nucleus to give rise to a new nucleus via the emission of gamma-ray.

5. Particle-particle reaction
In such a reaction, the projectile is absorbed by the target nucleus to give a compound nucleus which further breaks up to give the product nucleus.
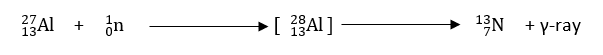
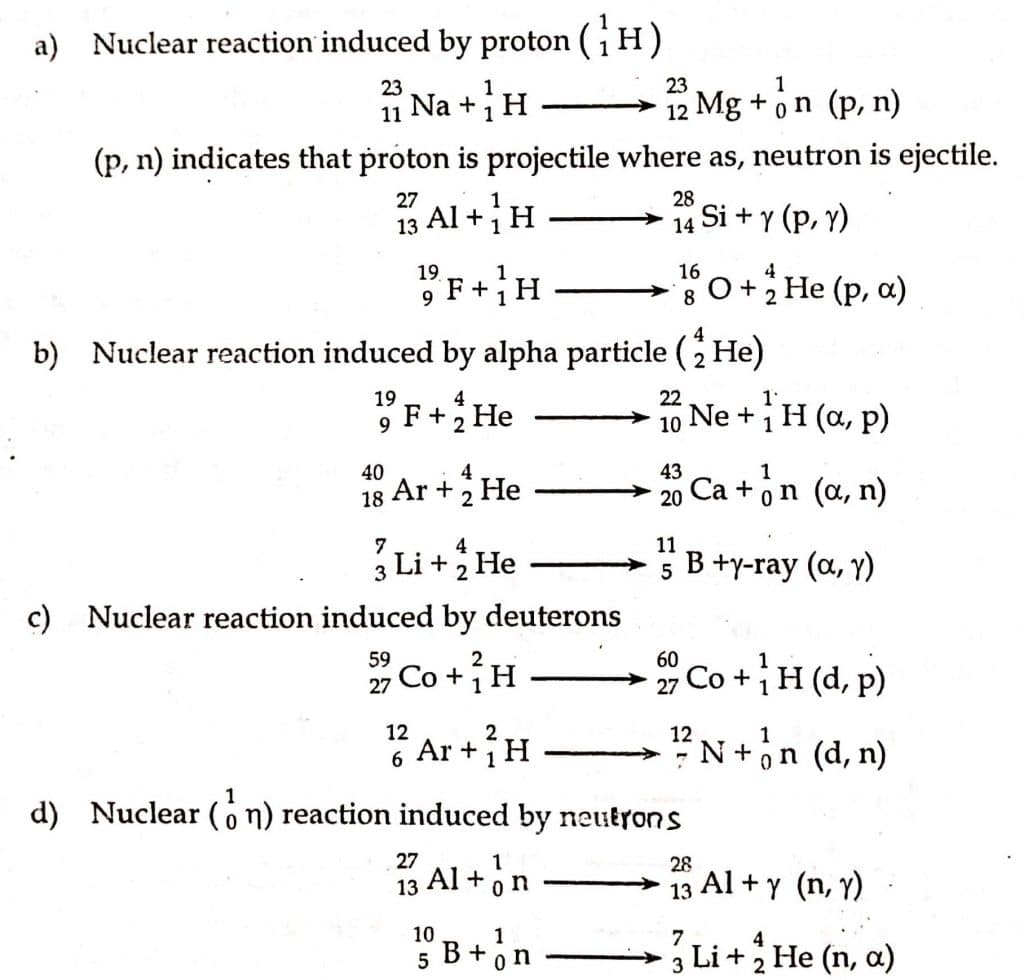
B. Nuclear reaction based on nature of bombarding particle
Based on it, nuclear reactions are of the following types:
- Nuclear reaction induced by proton (11H)
- Nuclear reaction induced by an alpha particle (24He)
- Nuclear reaction induced by deuterons
- Nuclear reaction induced by neutrons(10n)
Difference between chemical and nuclear reaction
| Chemical reaction | Nuclear reaction |
| Changes occur in the extranuclear region. | Changes occur in the nuclei. |
| Nucleons are rearranged; one nucleus gets converted into another. | Atoms are rearranged by the breaking and forming of bonds. |
| No new atomic species are formed. | New atomic species are formed. |
| Chemical reaction involves the absorption or release of a small amount of energy. | Involves absorption or release of a tremendous amount of energy. |
| Only valence electrons are involved. | Protons, neutrons, and other elementary particles are involved. |
| Can be controlled easily. | Cannot be controlled easily. |
| Mass loss during a chemical reaction is negligible. | There is measurable loss in mass during a nuclear reaction. |
Nuclear reactions video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






