Table of Contents
ToggleBalancing Redox equations by oxidation number method is quite famous and easy method used in balancing of reaction, and finding , understanding basic concept of oxidation number and valency. Oxidation number is generally man-made concept, while valency has a physical reality. An element’s valency is a number that is neither positive nor negative; in contrast, an oxidation number can be either.
Balancing redox equations by Oxidation Number method
For Balancing redox equation by oxidation number method, first we have to understand some basic terms. To put it simply, the Oxidation Number is the number assigned to each element in a chemical combination. The number of electrons that atoms in a molecule can gain, lose, or share while establishing chemical interactions with other atoms of a different element is known as the oxidation number. The proper method of balancing oxidation-reduction equation involving various steps, which are explain below.
- First of all we have to write the skeleton equation using correct formulas of those reactants and oxidants.
- Then, oxidation number of those atoms was kept which change in their oxidation state.
- Now, the change in ON of above atoms between final and initial oxidation states is find out.
- Next, the ions or molecules in the equation are multiplied by the appropriate coefficient such that the total ON rises and ON decreases of the ions are equal.
- In order to get RHS and LHS equal, the additional consistent coefficients are added using the conservation of matter.
- The net charges on both sides of the equation will be present if the equation is ionic and the reaction is occurring in an acidic medium. To balance charges, the right amount of hydrogen ions H+ is added to the right side.
- If more water molecules are needed to bring the amount of hydrogen and oxygen atoms on both sides of the equation to equal, they are added.
- When an ionic equation occurs in a basic medium, an adequate amount of hydroxide ions are supplied, but an equal amount of OH– ions must be added on both sides.
- If any molecules appears on the both sides of equation , we have to cancel them algebraically.
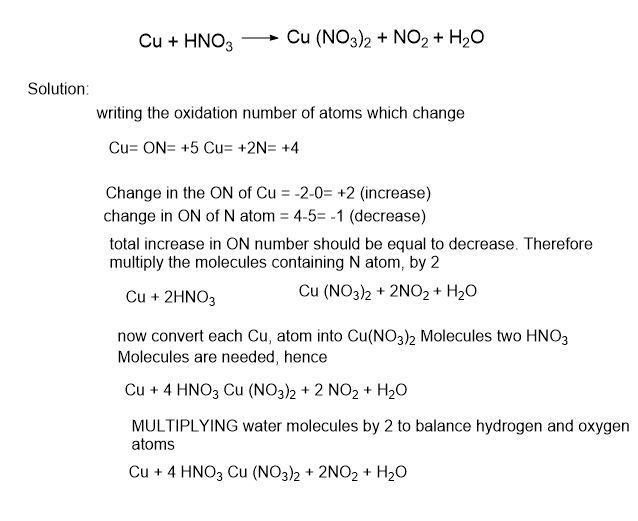
Lets explain it with an example:
- Balance the following equation by ON method
How to find the oxidation number of sulfuric acid H2SO4?
We are aware that oxygen O has a -2 charge and hydrogen H has a +1 charge. Sulfur S will balance the charges since the H2SO4 molecule is neutral. The equation now balances to 2 x +1 + S + 4 x -2 = 0. Solving this gives us +2 + S – 8 = 0S = +6. S in H2SO4 has an oxidation number of +6.