Table of Contents
ToggleGroup 13 elements, also known as the Boron family includes five elements; Boron (B), Aluminium (Al), Galium, Indium, and Thallium (Tl). They are distinguished by the presence of three electrons in the outermost shell of their atomic structure. They have a general valence electronic configuration of ns2np1 and +3 or +1 oxidation state; +3 being common while the heavier elements like Tl show a +1 oxidation state (inert pair effect).
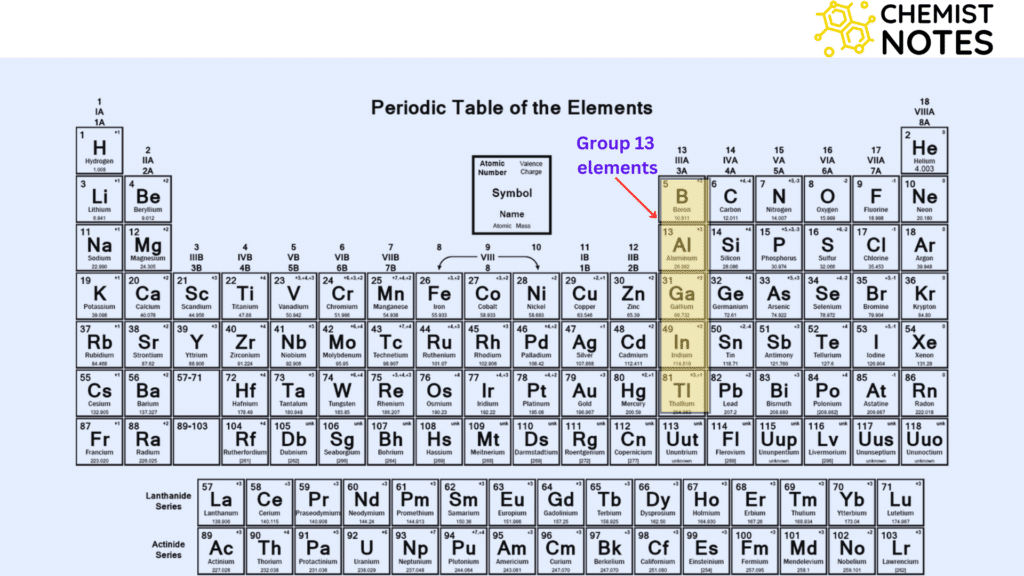
Electronic configuration of group 13 elements
| Element | Symbol | Electronic Configuration |
| Boron | B | [He] 2s2 2p1 |
| Aluminium | Al | [Ne] 3s2 3p1 |
| Gallium | Ga | [Ar] 3d10 4s2 4p1 |
| Indium | In | [Kr] 4d10 5s2 5p1 |
| Thalium | Thallium | [Ze] 4f14 6s2 6p1 |
General properties of group 13 elements
- Boron is a non-metal and always forms covalent bonds.
- There is no tendency to form univalent compounds.
- All BX3 compounds are electron deficient, thus forming a coordinate bond.
- The four elements; Al, Ga, In, and Tl all form trivalent compounds. These four elements are more metallic, and more ionic than B.
- Many of the compounds are covalent when anhydrous, but form ions in solution.
- The heavier member (Tl) shows an inert-pair effect.
Occurrence of group 13 elements
- Boron: It exists in nature along with oxygen and other natural elements but is not a naturally occurring element. Together with other elements found in nature, such as borates or orthoboric acids, it can produce a variety of compounds. These borates are widely distributed in soils, sedimentary rocks, and the oceans.
- Aluminum: On earth, aluminum does not exist in its free form. However, it is the most prevalent metal on earth and may be found in a variety of ores, including cryolite and bauxite. The main source of aluminum ore is bauxite.
- Gallium: Gallium is a rare mineral that can be found in small amounts in diaspore, bauxite, sphalerite, germanite, and coal.
- Indium: Indium, like gallium, is a rare element that occurs in only 0.1 parts per million on Earth.
- Thallium: We can obtain thallium from the ores of zinc, lead, or copper. It is also present in ore pyrites, which are useful in the production of sulphuric acid. It is usually obtained as a by-product of copper, zinc, and lead refining
Physical properties of group 13 elements
Atomic radius
As you move down the group, the addition of one more shell causes the atomic radius to increase. The atomic radius of Ga in group 13 elements, however, is less than Al. This is due to the presence of d-orbitals in Ga. The shielding effects of these greatly dispersed d-orbitals are poor, thus leading to a decrease in the atomic radium with an increase in the nuclear charge. Among the elements in group 13, boron has the smallest atomic radius.
Density
The group 13 elements are denser than the group 2 elements. This is due to small sizes, which result in smaller volumes. The density of group 13 elements increases as we move downward the group from B to Al.
Melting point and Boiling point
Boron has the greatest boiling point of the group 13 elements, whereas gallium has the lowest. The lowest melting point metal, gallium, is typically liquid in the summer. Because it is smaller than other atoms, boron forms strong covalent bonds with them. The atoms are consequently tightly packed in the solid state. Therefore, compared to all other group 13 elements, boron has the highest melting and boiling values.
Oxidation state
The elements of group 13 generally display oxidation states of +3 and +1. The elements all have three outer electrons. Apart from Tl, they normally exhibit +3 oxidation state. The ability to generate a +1 ion increases as we move downward the group due to the inert pair effect.
Let us consider B3+ and B+. Experimental evidence suggests that B3+ is more stable than B+. Now think about Tl3+ and Tl+. Tl3+ was shown to be less stable than Tl+. This can be explained via the inert pair effect. Due to the insufficient shielding provided by the intervening electrons, the s-orbital does not participate in chemical bonding.
Ionization energy
The ionization energy value does not decrease smoothly down the group. The decrease from B to Al is the usual trend for descending a group associated with increased size. The poor shielding by d electrons and the resulting d block contraction affect the values for the later elements.
Electropositive character
The metallic nature or electropositive character of the elements increases from B to Al, but then decreases from Al to Tl.
Electronegativity
From B to Al, the electronegativity first decreases, and from Al to Tl, it slightly increases. The poor shielding of the d and f orbitals is responsible for this.
Chemical properties of group 13 elements
Reaction with oxygen
At high temperatures, all group 13 elements react and produce M2O3 trioxides.
4M (s) + 02 (g) → 2M203(s)
Ti2O is formed when Ti and oxygen react. Additionally, Ti2O3 is another substance that is produced. As you move down the group, group 13 components begin interacting with oxygen more vigorously.
When present as boron crystals, oxygen does not react with them. When heated, amorphous boron that has been finely divided reacts with oxygen to form B2O3. Thermodynamically, aluminum ought to react with air, yet it doesn’t. In this case, it is a result of Al203 forming a protective coating on the metal’s surface, making it inert.
Reaction with acids and alkalis
Boric acid is produced when boron reacts at higher temperatures with strong oxidizing acids, such as a highly concentrated solution of H2SO4 and HN03, but not with non-oxidizing acids, such as HCl.
B(s) + 3HNO3 (aq) → H3BO3 (aq) + 3NO2 (g)
Up to 773 K, alkali (NaOH and KOH) do not react with boron. It produces borates if the temperature is raised higher.
2B (s) + 6K0H (s) → 2K3B03(s) + 3H2(g)
The remaining elements in group 13 react with both oxidizing and non-oxidizing acids, producing hydrogen gas as a result.
Reaction with water
Water and steam do not react with boron, however, it does react with steam at extremely high temperatures.
2B + 3H2O → B2O3 + 3H2
Without an oxide layer, aluminum decomposes cold water into hydrogen gas. Only in the presence of oxygen gas would gallium and indium react with water. Thallium emits TiOH when in humid air.
4Tl + 2H2O + O2 → 4TlOH
Reaction with halogens
At very high temperatures, they undergo a reaction with halogens to form trihalides MX3. As a result, Ti simply forms TiF3 and TiCl3.
2M(s) + 3X2 (g) → 2MX3
Reaction with metal
Borides are formed when boron reacts with metals. The remaining elements in Group 13 are uncertain of combining with metals. This illustrates the non-metallic nature of boron.
3Mg + 2B → Mg3B2