Table of Contents
ToggleAn activated complex is a state that forms during the conversion of reactants into products. According to the theory of transition states, most reactions result in the formation of an intermediate species known as an activated complex or transition state that involves energy changes. Sometimes, reactant particles undergo collision and are unaffected. At times, some collisions result in the production of products. The term “activated complex” refers to the state of the particles that are situated between reactants and products.
A temporary atomic arrangement at the top of the activation energy barrier is known as an activated complex. The activated compound only lasts for a very short time (about 10-13s) due to its tremendous energy. There is an equal chance that the activated complex will either continue to generate products or return to the initial reactants.
How Activated Complex Work?
Suppose a chemical process that results in the formation of products C and D from reactants A and B. In order to generate the products, the reactants must collide and interact. Increasing the temperature, increasing the concentration of reactants, or introducing a catalyst all enhance the likelihood that A and B will collide. A and B produce the complex A-B in a reaction with an activated compound. The compound can only form if there is enough energy (the activation energy).
The activated complex has a higher energy than either the reactants or the products, making it unstable and only transitory. If there is insufficient energy to generate the products, the activated complex eventually breaks down into the reactants. The products form if enough energy is present.
Mechanism of Activated Complex
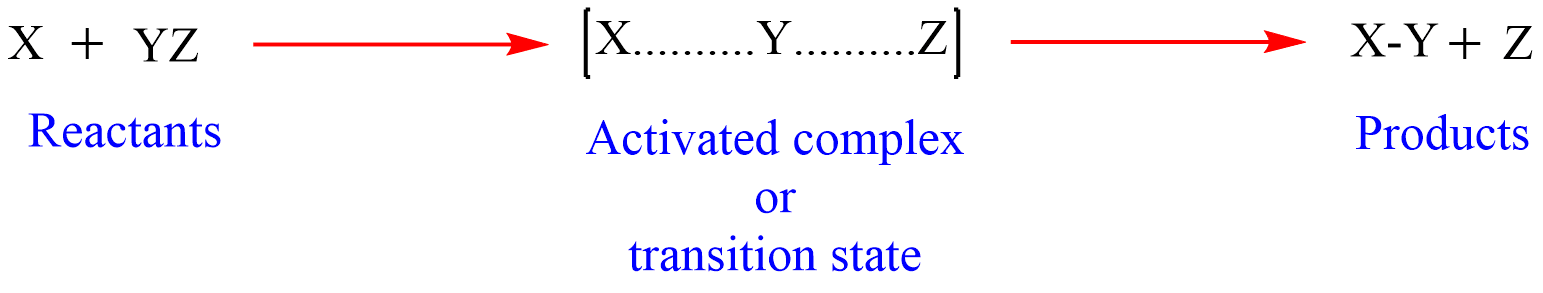
In the beginning, both X and Y-Z pose a certain potential energy. But the approach of X to YZ molecule causes an increase in potential energy to a critical value, so the Y-Z bond is weakened. As X approaches Y-Z and as it draws nearer to Y, Z starts being repelled from Y until a state is reached in which both X and Z are loosely attached to Y and are equidistant from it. In this stage, the species called activated complex of type X……….Y……….Z is formed. This species is very unstable because of high energy, has a very short life, cannot be isolated, and changes to the stable product X-Y by expelling Z.
The activation of the complex (or transition state), according to the kinetic model, is the result of sufficiently intense collisions between the reactant molecules. Kinetic energy is transformed into potential energy during chemical reactions through bond stretching, partial bond breakage and formation, angular distortion, and other processes. The specific reaction determines the exact process. The transition state (T.S.) is the species with the highest possible potential energy as a result of molecular species colliding and moving. Activation energy (Ea) is the amount of energy needed to transition from the reactant ground state to the transition state. In other words, activation energy is the amount of energy needed to move from the reactant ground state to the potential energy barrier.
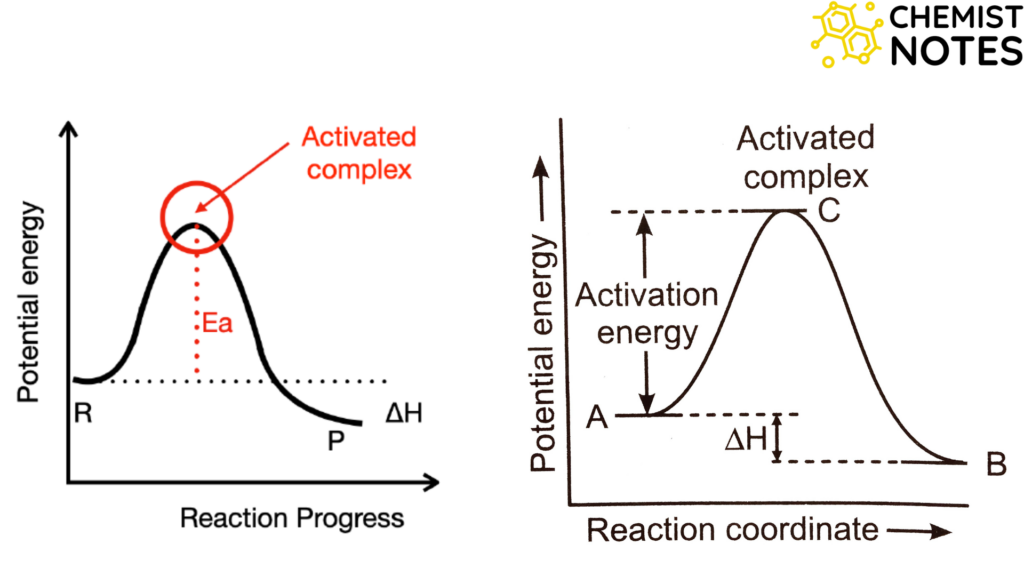
After the T.S., the so-formed collection of atoms rearranges towards a more stable species where old bonds are fully broken and new bonds are fully formed. Thus, geometric rearrangement towards a more stable configuration (relaxation) occurs. There are many reactions that take place in more, than a single step. In multistep reactions, a new T.S. is reached for each distinguishable step.
The two-dimensional plot of the energy system as it moves along the reaction coordinate is called the reaction profile. The reaction profile supplies a qualitative model and experiments are designed to show what reacting species are present in T.S. Also whether the intermediate exists or not. The reaction rate is determined by the energy required to overcome the energy barrier i.e. to reach the T.S. The higher the barrier, the smaller the fraction of the reactant molecules that will have energy equal to Ea at a given temperature, and thus slower the reaction and vice-versa.
Difference Between Activated Complex and Transition State
Although the terms “activated complex” and “transition state” are sometimes used synonymously, they have very different meanings. Some of the major differences are:
| Activated Complex | Transition State |
| A group of intermediate molecules formed during the course of a chemical reaction is referred to as an activated complex. | The transition state is the intermediate of a chemical reaction with the greatest potential energy. |
| The activated complex can be found anywhere around the transition state. | The transition state is the only molecular arrangement that may be found at the peak of the reaction’s energy map. |
| The potential energy of an activated complex is greater than that of the reactants. | Among the intermediate structures, the transition state has the most potential energy. |
| The activated complex can either generate the reaction’s end product or move backward, generating reactants but not products. | The transition state has a high probability of generating the product rather than the reactants again. |