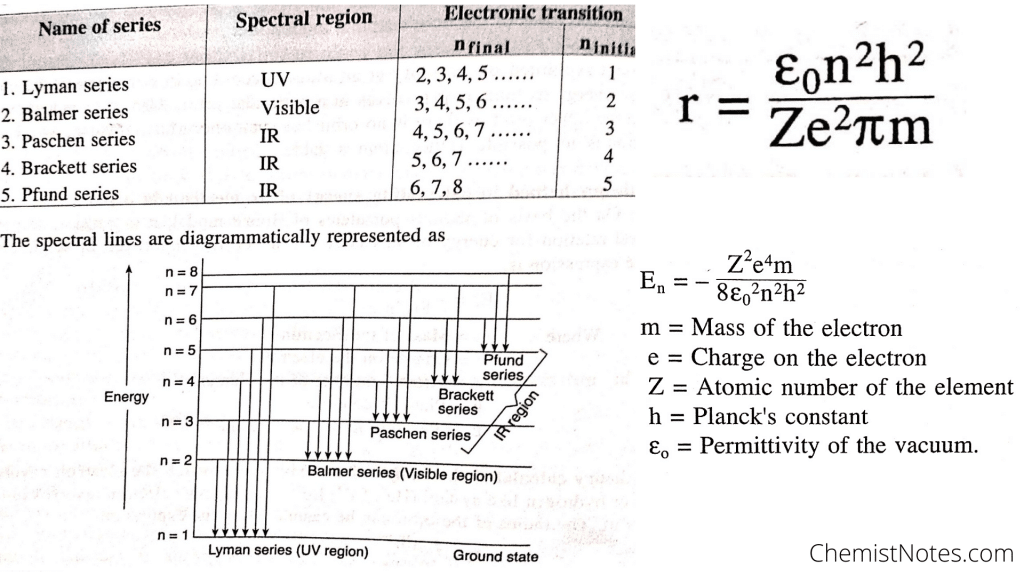
Table of Contents
ToggleIn 1913, Danish physicist Neil Bohr proposed the Bohr atomic model based on Planck’s quantum theory of radiation. This atomic model is the modification of Rutherford’s atomic model (the nucleus is positively charged and is surrounded by electrons (negatively charged particles). According to Bohr’s atomic model, “a positively charged nucleus is surrounded by electrons moving in fixed orbits.” Later on, He was also awarded by Nobel prize in physics for his contribution to the structure of atoms.
Postulates of Bohr’s model of atom
Bohr’s atomic model explains the structure of atoms, basically of the Hydrogen atom, and is therefore also called Bohr’s model of Hydrogen atoms. Bohr’s atomic model suggests that electrons surround the nucleus moving in orbit like planets around the sun. The main postulates of Bohr’s model of the Hydrogen atom are:
- In an atom, electrons revolve around positively charged nucleus in a fixed cicular path called orbits or shells. The orbits are reprsented as 1, 2, 3, …..etc., and the shells are represented by K, L, M, N,………..etc.
- The energy of the electrons in a particular energy level remains constant, and hence accounts for the stability of an atom.
- Only those orbits are permitted whose angular momentum is the integral multiple of h/2π i.e. mvr = nh/2π. This postulates suggest that electron’s angular momentum is quantized
- An elctrons jumps from higher energy level to lower energy level when it absorbs energy, while looses energy when the transition occurs from higher energy level to lower energy level. The difference in energy between two energy state is hv i.e. (E2-E1 = hv).
Merits of Bohr’s model of atom
- Bohr’s atomic model explains the stability of an atom on the basis that electrons energy remain constant in an orbit or shell.
- It gives basis to find energy of electron in a particular orbit, and the radius of an orbit from which electron revolves around positively charged nucleus.
- Bohr’s atomic model succesfully explain the atomic spectra of Hydrogen atom.
Limitations of Bohr’s model of Hydrogen atom
Some of the failures or limitations of Bohr’s atomic model are:
- Bohr’s atomic model fails to explain Stark effect (splitting of spectral lines under the influence of magnetic field) and Zeeman effect (splitting of spectral lines under the influence of electric field).
- This theory violates Heisenberg Uncertainty Principle.
- Fails to explain spectra for multielectron systems/largerr atoms
- Fails to explain the shape of the molecule, and so on.
Difference between Rutherford and Bohr atomic model
| Rutherford Atomic Model | Bohr’s Atomic Model |
| It states that the nucleus is positively charged and is surrounded by electrons (negatively charged electrons | According to Bohr’s atomic model, “a positively charged nucleus is surrounded by electrons moving in fixed orbits.” |
| It is based on classical theory. | It is based on quantum theory. |
| It couldn’t explain the stability of the atom. | It accounts for the stability of an atom. |
| It is based on an alpha particle scattering experiment. | It is based on the atomic spectra of the Hydrogen atom. |
| It doesn’t explain the orbits or energy level. | It successfully explains that electrons move in permitted orbit with fixed energy and angular momentum. |
Bohr’s Atomic model video
FAQs/MCQs
How did Niels Bohr describe electrons in his atomic model?
According to Bohr’s atomic model, “a positively charged nucleus is surrounded by electrons moving in fixed orbits.” Electrons revolve around the nucleus in a fixed circular path called an orbit.
how is Bohr’s atomic model different from rutherford’s model?
Bohr’s atomic model is different that Rutherford’s atomic model in the sense that Bohr’s atomic model is based on quantization of energy and angular momentum of electrons. According to Bohr’s theory, electrons revolve around the nucleus in permitted orbits with a fixed amount of energy and angular momentum. The existence of line spectra describes that energy is quantized.
where are the electrons found in Bohr’s atomic model?
In Bohr’s atomic model, electrons revolve in a fixed orbit around the nucleus.
how did Bohr expand on rutherford’s model of the atom
Bohr’s atomic model is the modification/expansion of Rutherford’s atomic model. Bohr used the concept of quantization of energy and angular momentum of the electrons and successfully explains the atomic model.
carbon atom Bohr model
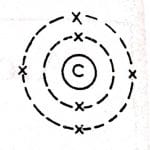
Carbon has 6 neutrons and 6 protons, and the nucleus is surrounded by electrons moving in the K shell and L shell.
Bohr model of an oxygen atom
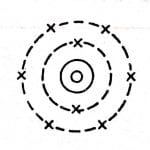
Oxygen has 8 neutrons and 8 protons, and the nucleus is surrounded by electrons moving in the K shell and L shell.
Bohr model of sodium atom
Sodium has 12 neutrons and 11 protons, and the nucleus is surrounded by electrons moving in the K shell and L shell.
beryllium atom Bohr model
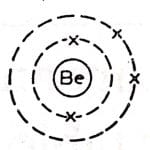
Beryllium has 5 neutrons and 4 protons, and the nucleus is surrounded by electrons moving in the K shell and L shell.






