Table of Contents
ToggleMetallurgy is the process of extracting a metal in pure form from one of its ore and utilizing them for useful purposes. This method used depends upon the nature of ore, the properties of the metal, and the local conditions. Thus, it is not possible to have a universal method for the extraction of all metals from their ores.
Introduction to Metallurgy
Metallurgy is a branch of materials science and engineering that focus on the studies of the physical and chemical behavior of metallic elements. The science and technology of metals, or how science is applied to the production of metals, as well as the engineering of metal components used in products for both consumers and manufacturers, are all included in the field of metallurgy.
A process used to extract metals in their pure form is referred to as metallurgy.
Types of Metallurgy
Metallurgy is further divided into two broad categories:
- Chemical metallurgy
- Physical metallurgy
Chemical metallurgy
This is primarily concerned with metal reduction and oxidation, as well as metal chemical performance. Mineral processing, metal extraction, thermodynamics, electrochemistry, and chemical degradation are all topics of study in chemical metallurgy.
Physical metallurgy
On the other hand, it focuses on metals’ mechanical properties, physical properties, and physical performance. Crystallography, material characterization, mechanical metallurgy, phase transformations, and failure mechanisms are some of the topics studied in physical metallurgy.
Terms involved in Metallurgy
Minerals
The naturally occurring material in the earth contains metal in nature from (free state) or in the combined state (as compound).
A large number of metals occur in nature in the combined state with other elements but some less reactive metals such as Ag, Au, Pt, etc. occur in the natural form (elemental form).
Bauxite: [Al2O3.2H2O]
Clay: [Al2O3.2SiO2.2H2O]
Bauxite and Clay are the ores of aluminum.
The mineral is pure inorganic homogenous substances found in nature.
Ores
The mineral from which the metals can be conveniently and economically extracted are ores. Thus, all ores are minerals but all minerals are not ores.
For example, Both Bauxite [Al2O3.2H2O] and clay [Al2O3.2SiO2.2H2O] are minerals of Aluminum. But for the extraction of Aluminum Bauxite is used not clay hence, bauxite is an ore of Aluminum.
Gangue or Matrix
The unwanted earthy, rocky, and silicious impurities present in ores are called gangue or matrix. Example: Sand, rock, clay, etc. are associated with ore. These are mostly infusible and chemically acidic-SiO2, Basic-CaO, FeO, and MnO.
Flux
These are additional or foreign materials used to remove gangues or matrices. These are mostly fusible and chemically acidic- SiO2, P2O5; basic- CaO, FeO, MnO, MgO; Neutral: F-S and Cl-S
Slag
Lighter and fusible products are obtained by the chemical reaction between gangue and flux are called slag.
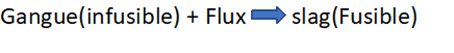
Principles of Metallurgy
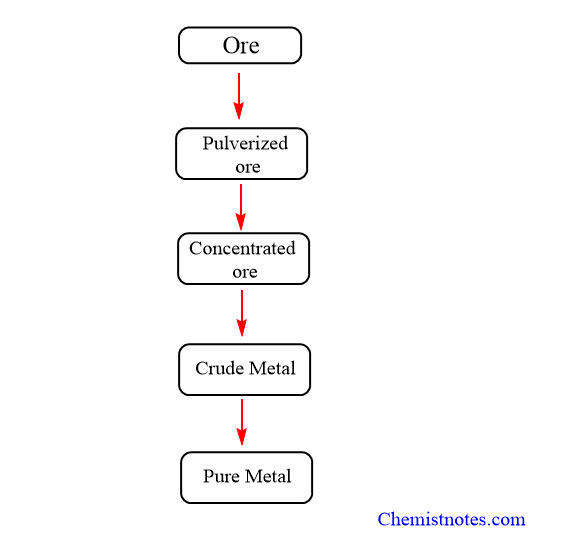
1. Crushing and Pulverization
The ore is typically obtained in the form of large rock fragments. These large ore lumps are crushed into smaller pieces using jaw crushers and grinders. The ore is crushed and then pulverized (powdered) in a stamp mill. The heavy stamp rises and falls on a hard die, powdering the ore. A ball mill can also be used to pulverize materials.
2. Concentration or dressing of ores
The technique used to increase the percentage purity of ores of metals by removing the gangue or matrix (impurities) present in the ores is concentration. The concentration of ore is done by physical as well as chemical methods.
2.1 Physical Methods
Based on the nature of ore, methods are generally employed for further process.
2.1.1 Gravity Separation or Levigation or Hydraulic washing
- Finely powdered ore is washed with a jet or steam of water which removes the water-soluble and lighter impurities.
- Wilfey table and Hydraulic press techniques are used in this process.
- Based on the difference in specific gravity of the gangue and the ore particles.
- Oxides and carbonate ores are concentrated by this method. Example: Haematite
- Soluble ores are not concentrated. For example, Carnalite.
2.1.2 Electromagnetic Separation
- Based on the magnetic property of either ore or impurities.
- Finely powdered ore is brought to a powerful magnet by which magnetic and non-magnetic particles are separated (by a revolving magnet)
- Magnet particles are taken as ores for Fe, Co, Ni, etc. and non-magnetic particles are taken as ores for Cr, Mn, W, etc.
- Ferromagnetic ores are concentrated by this method. Eg. Wolframite (FeWO4) a magnetic ore is separated from non-magnetic ore cassiterite (SnO2) by this method.
2.1.3 Froth floatation process
- This process is used for sulfide ores.
- Separation is based on the wetting of ore particles by oil (frothing agent or frothers) and the gangue particles by water.
- The water in the tank is stirred as a result, causing the sulfide ore particles to adhere to the oil and rise to the surface as froth.
- Gangue particles settle at the bottom of the water tank because they are heavier.
- The separated froth is used to extract concentrated sulfide ore.
2.2 Chemical Methods
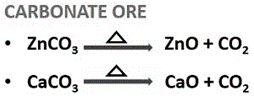
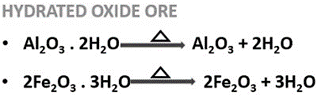
2.2.1 Calcination
- It is a chemical separation method for carbonate or hydrated oxide ores.
- It involves heating the ore below its melting point (fusion temperature) in absence of air.
- This process makes the ore porous and is performed in a reverberatory furnace.
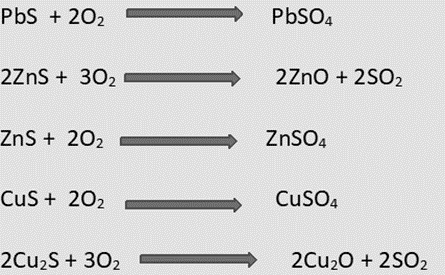
2.2.2 Roasting
- To undergo chemical changes, ores (typically sulfide ores) are subjected to roasting, a process in which they are heated and dried at temperatures below their melting points, either alone or in combination with other materials (additives).
- Calcination is similar to roasting, but this process is concerned with the changes caused by the expulsion of some ingredients such as water and carbon dioxide.
- Chemical changes such as oxidation, chlorination, and so on occur during roasting.
- Roasting is typically done in a reverberatory furnace or a blast furnace.
2.2.3 Leaching
- To make the ore soluble while keeping impurities insoluble, it must be treated with a suitable reagent. With the help of the proper chemical process, the ore is extracted from the solution.
- Impurities in bauxite ore include ferric oxide, titanium oxide, and silica.
- The alumina dissolves to form soluble sodium meta-aluminate when the powdered ore is digested with an aqueous solution of sodium hydroxide at about 150°C under pressure, but the ferric oxide, TiO2, and silica remain as insoluble components.
- Au and Ag are also extracted from their native ores by leaching (Mac-Arthur Forrest Cyanide process).
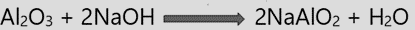
The following methods are used for the reduction of calcined or roasted ores into the free metals
3. Reduction (Extraction of metals)
Smelting
- It is a process that involves melting in which a metal is separated from its ore.
- It is usually done in a controlled air supply reverberatory furnace or blast furnace.
- Sodium, magnesium, and aluminum are some of the reducing agents used.
- The calcined or roasted ore is heated in a reverberatory or blast furnace with carbon (coal or coke).
- The oxide is reduced to metal by the carbon and carbon monoxide is produced by incomplete combustion of carbon.
- Flux is the substance used in smelting to convert infusible impurities into fusible impurities, and slag is the fusible mass.
- Basic flux is used for acidic impurities and acidic flux is used for basic impurities.

Out of the different processes of smelting, carbon reduction and aluminum reduction are important processes:
a. Carbon-reduction process (Smelting):
- The roasted or calcined ore is mixed with coke (C) as a reducing agent, and a suitable flux is fed into a blast furnace by hot air above the melting point of ore.
- As a result, the oxide is reduced to metal, and the flux forms a slag with impurities.
- Molten metal is obtained at the bottom of the furnace, and slag floats over it.
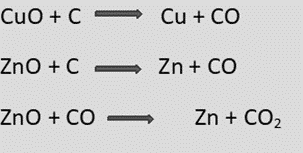
b. Reduction by Aluminum (Aluminothermic or Gold-Schmidt alumino thermic process)
- Due to oxygen’s greater affinity for certain metals than carbon, such as CrO3 and MNO4, some metallic oxides like these cannot be reduced by carbon and hence, Aluminum is employed as a reducing agent.
- In a crucible, the mixture of roasted or calcined ore and aluminum known as thermite is combined with barium peroxide and the appropriate flux.
- Magnesium is introduced into the crucible for ignition, releasing a large amount of energy; metallic oxide is reduced to metal in molten form and collected at the bottom.

Electrolytic Reduction
- This method is used to extract metals that are highly electropositive metals (alkali and alkaline earth metals).
- The fused ore is electrolyzed, and metal is deposited on the cathode by reduction.
- In order to lower the melting point of the ore, other materials may occasionally be added.
4. Purification or Refining of Metals
4.1 Liquation
- Metals with low melting points (Pb, Sn, etc.) can be purified using this method.
- Impure metal is placed in a furnace’s inclined bed and heated.
- The metal melts and flows away, leaving non-fusible impurities (oxides) behind.
4.2 Distillation
- This process refines metals with low melting points (Zn, Hg, Cd).
- In this method, the metal to be purified is heated to around its boiling point, causing the metal to vapourize and leave impurities behind.
- Metal vapors are condensed to produce pure metal.
4.3 Pyrometallurgical oxidation process
- This method is used for easily oxidizable impurities with greater affinity with oxygen than the metal itself.
- Impure metal is heated in an open furnace with an excess of air.
4.3.1 Tossing
- The crude metal is taken in a small ladle and tossed from a height in the air.
- Impurities thus come in contact with air and get oxidized.
4.3.2 Puddling
- The crude molten metal is stirred with a long dry wooden bar, and the impurities like C, P, and S, which come in contact with air get oxidized.
4.3.3 Cupellation
- Cupellation process is used in the metallurgy of refining those metals which contain the impurities of other metals forming volatile oxides.
4.3.1 Bessemerization
- Impurities that are not metallic are oxidized and removed as waste gas.
4.4 Zone Refining (Fractional Crystallization)
- Elements such as Si, Ge, Ga, etc. which are used as semiconductors are refined by this method.
- Metals of a high degree of purity are obtained.
- Based on the difference in solubility of impurities in the molten and solid state of the metal.
4.5 Electrolytic refining
- Elements such as Ag, Au, Cu, Al, etc . are refined by this method.
- Anode is made of impure metal, and cathode is made of pure metal.
- An electrolyte is made from a salt of the same metal.
- When electricity is applied, the metal dissolves from the anode and deposits on the cathode.
- Soluble impurities pass into the solution, while insoluble impurities remain at the anode.
4.6 Poling
- Hydrocarbons in greenwood poles reduce metallic oxide (impurities) into free metal when molten metal is stirred with them.

Flow sheet diagram for metallurgical operations
Metallurgy Video
FAQs
Powder metallurgy
Powder metallurgy is a process to form metal performed When compacted metal powders are heated to just below their melting temperatures.
Calcination is used in metallurgy for removal of
calcination is used in metallurgy for removal of carbonate or hydrated oxide ores.