Table of Contents
ToggleThe study of the rate equation for radioactive disintegration is based on the following two laws:
- All radioactive elements’ atoms spontaneously disintegrate, which causes them to emit α, β, and γ-rays as they break apart to produce new radioactive products.
- The rate of disintegration is determined by the law of chance rather than by external variables like temperature, pressure, chemical environment, etc. The amount of radioactive element present is exactly related to the amount of radioactive element that disintegrates in a time period.
Rate equation of radioactive decay
As per the radioactive disintegration of a different element, the quantity of a radioactive element that disappears in unit time (rate of disintegration) is directly proportional to the amount present. Thus, the radioactive disintegration follows first-order kinetics.
When there are N radioactive atoms present at any time, the rate of disintegration is expressed as -dN/dt, where dN is the number of radioactive atoms that disintegrate during the time interval dt. The negative sign indicates the decrease of atoms present with an increase in time. Hence,

Where ‘k’ is the proportionality constant called radioactive constant or disintegration constant.
On rearranging the above equation,

Integrating equation (ii), we get
-ln N = kt + C ………………………….(iii)
Where ‘C’ is an integration constant
When t = 0, N = No , thus equation (iii) becomes,
C = -ln No …………………………………..(iv)
From equations (iii), and (iv), we get
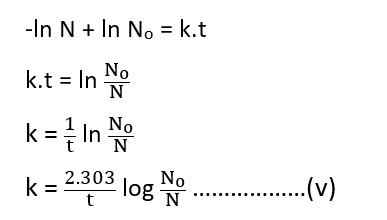
This equation (v) is the rate law equation for radioactive disintegration.
Disintegration constant (k)
The radioactive constant or disintegration constant or popularly called decay constant is the ratio of the amount of the substance which disintegrates in a unit of time to the amount of substance present. It depends only on the nature of the radioactive substances rather by any physical or chemical state.
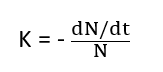
The unit of a radioactive constant is time-1.
Rate equation video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






