Table of Contents
ToggleNuclear fission and fusion are such types of nuclear reactions in which the composition of certain nuclei undergo change to form an atom of a new element. Nuclear fission involves the splitting of heavy nuclei into lighter nuclei, while nuclear fission is the process by which lighter nuclei combine to form a heavy stable nucleus. Such nuclear reactions release a tremendous amount of energy.
Nuclear fission
Nuclear reaction in which a heavy nucleus (235U, Th, Ra) when interacting with a bombarding particle (10n) breaks up into two or more lighter nuclei of nearly equal size/mass is called nuclear fission reaction. A tremendous amount of energy is liberated in this process.
The heavier nuclei are unstable and frequently disintegrate to form nuclei with intermediate atomic weights because of the low binding energy per nucleon. However, these reactions are brought about by high-energy particles like slow neutrons. Nuclear fission is a type of reaction mechanism wherein heavier nuclei are divided into lighter nuclei and simultaneously release a significant quantity of energy when they are bombarded with high-energy particles.
Example: When 92235U is bombarded with slow-moving neutrons, it breaks up into two fragments 56144Ba and 3690Kr, and a number of neutrons.
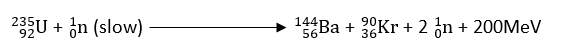
The neutrons released can further attack other uranium atoms causing fission, and hence nuclear fission reaction is a chain reaction.
Controlled Nuclear Fission
A controlled nuclear reaction is carried out for the continuous production of energy. In this reaction, the speed of neutrons is decreased by mixing fissionable material with moderators such as graphite or heavy water. These slow down moving neutrons and collide with a nucleus and further fission takes place at a controlled rate.
For example: When the slow-moving neutrons collide with 92235U nucleus, a fission reaction takes place and more neutrons are produced. When these pass through carbon and hit new 92235U nuclei, they are once again slowed down, causing fission and neutrons.
Uncontrolled Nuclear Fission
In such a reaction, nuclear released from the reaction further strike nuclei to cause more fission with the release of more energy and more neutrons. These neutrons undergo further fission in an uncontrolled manner until the whole uranium (U-235) gets finished. A huge amount of energy is liberated in this process. This principle is used to make an atom bomb.
Nuclear Fusion
The lighter nuclei have low binding energy and hence are less stable. Thus, two or more lighter nuclei have a tendency to combine or fuse together to give the nucleus of higher atomic mass and binding energy resulting in higher stability. The nuclear reaction in which two or more lighter nuclei combine with each other to form a stable nucleus of higher stability is called a nuclear fusion reaction. A tremendous amount of energy is released in this process.
When highly accelerated protons, neutrons, etc. are allowed to strike the nuclei of lighter elements, a reaction like this occurs. The positively charged atomic nuclei oppose one another and prevent easy fusion as a result. It is also known as a thermonuclear reaction since a very high temperature (>106 K) is necessary to overcome the electrostatic attraction between the two nuclei that are undergoing fusion. Only the interior of stars has the high temperature needed to initiate fusion. However, the fusion of 92235U can result in the high temperature needed for fusion.
The nuclear fusion process, like nuclear fission reactions, is accompanied by a mass defect that causes a huge quantity of energy to be released. Some of the examples of nuclear fusion reactions are:
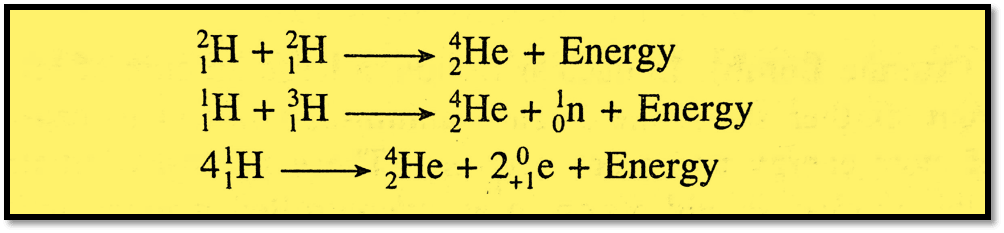
A hydrogen bomb is based on the principle of nuclear fusion reaction.
How are nuclear fission and nuclear fusion similar?
Both nuclear fission and fusion involve nuclear reactions that release large amounts of energy. However, their principles are different. Nuclear fusion is the process by which two light nuclei combine to form heavy nuclei, whereas nuclear fission is the breaking of heavy nuclei into two lighter nuclei.
What is the difference between nuclear fission and nuclear fusion?
Some of the major differences between nuclear fission and fusion are:
| Characteristics | Nuclear Fission | Nuclear Fusion |
| Definition | Nuclear fission is the process of splitting heavy nuclei into two lighter nuclei. | Nuclear fusion is the process by which two light nuclei combine to form heavy nuclei |
| Reactions | Involves chain reaction | Chain reaction is not involved |
| Occurence | Do not occur naturally. | Occurs in stars, such as the sun |
| Conditions | High-speed neutrons are required to cause nuclear fission. | High temperature and pressure are required. |
| Reactions nature | Can be controlled | Cannot be controlled. |
| Byproducts | Highly radioactive particles are produced | Fusion reactions don’t typically produce radioactive particles, but they can if a fission “trigger” is applied. |
| Energy required | A small amount of energy is required to split into two atoms. | High energy is required to combine two atoms together. |
| Energy released | The energy released during nuclear fission is less than that in the nuclear fusion process. | The energy released is almost 3 to 4 times greater than the energy liberated in fission. |
| Consequences | Nuclear fusion produces radioactive wastes, and hence its disposal is a serious issue. | Do not produce radioactive wastes. |
| Nuclear weapon | Atom bomb is based on the principle of nuclear fission. | Hydrogen bomb is based on the principle of nuclear fusion. |
Nuclear fission and fusion video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






