Table of Contents
ToggleAtomic fluorescence spectroscopy is an analytical quantitative technique employed for the determination of a concentration of elements present in a sample. It is used mostly in the analysis of more than 65 metals in biological samples, agricultural samples, pharmaceuticals, environmental, water, and industrial oils.
Principle of Atomic Fluorescence Spectroscopy
Atomic fluorescence spectroscopy (AFS) is the emission of radiation energy in the UV-visible region from gas-phase atoms that have been excited to higher energy levels by absorption of radiant energy. Usually, a flame is used to obtain the atom in a gaseous state. It is a radiative emission process that proceeds from the lowest singlet (S1) to the singlet ground state(S0).
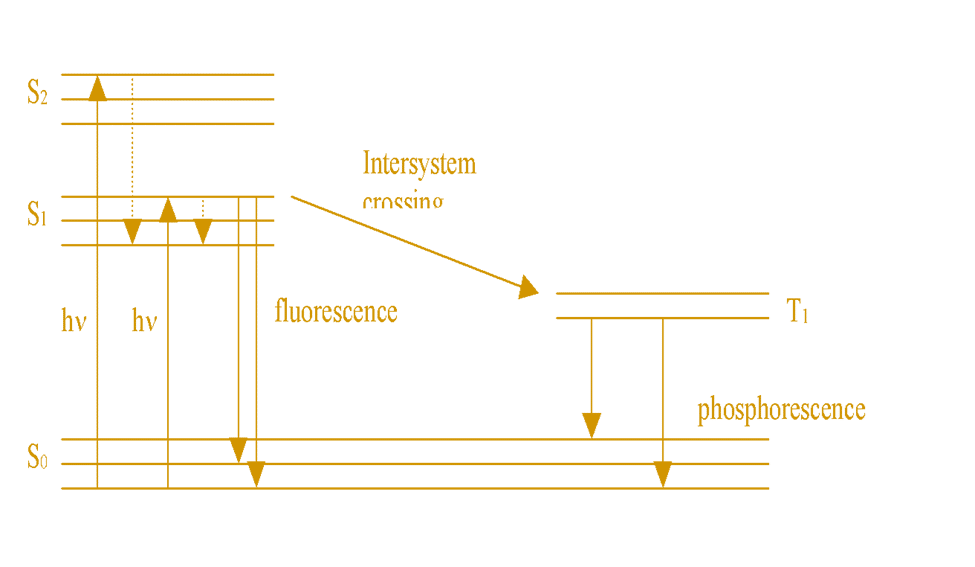
In simple words, when a sample is introduced into flame or other radiation sources, it absorbs photons and gets excited to a higher level. The atoms at an excited state are unstable and hence return to the ground state by emitting a photon. This emitted photon is used to determine the concentration of elements present in a sample. The relationship of concentration to the intensity of fluorescence is calculated from the Beer-Lambert law.
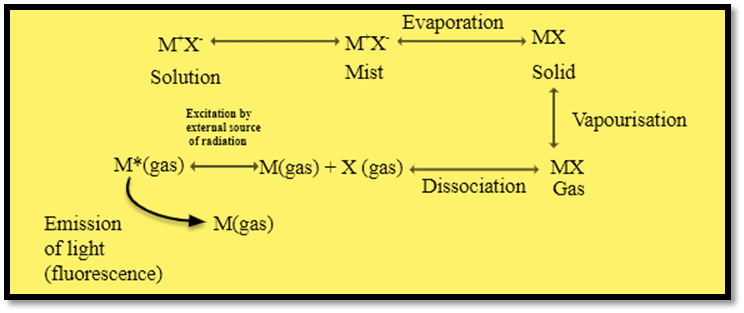
When a solution containing a suitable compound of the metal to be investigated is aspirated into a flame (non-flame technique can also be used for atomization), the following events occur in rapid succession.
- Evaporation of solvent leaving a solid residue
- Vaporization of the solid with dissociation into its constituents atoms, which initially will be in the ground state.
- Absorption of radiant energy from the external source of radiation.
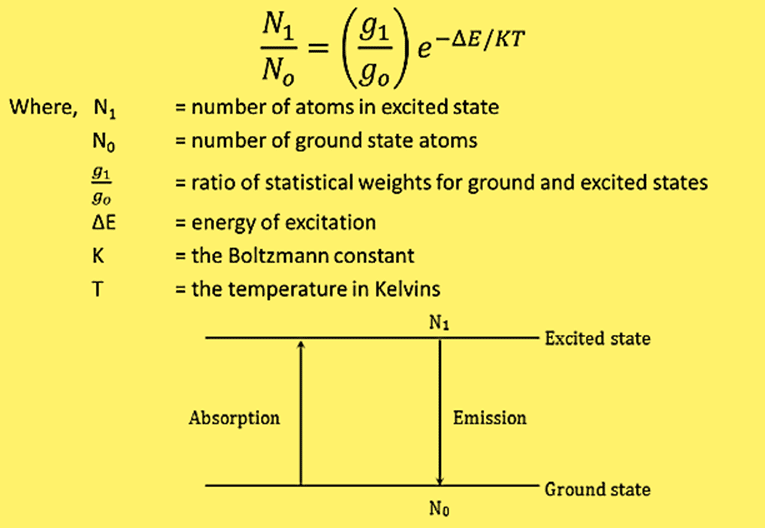
The relationship between the ground state and excited state population is given by the Boltzmann equation.
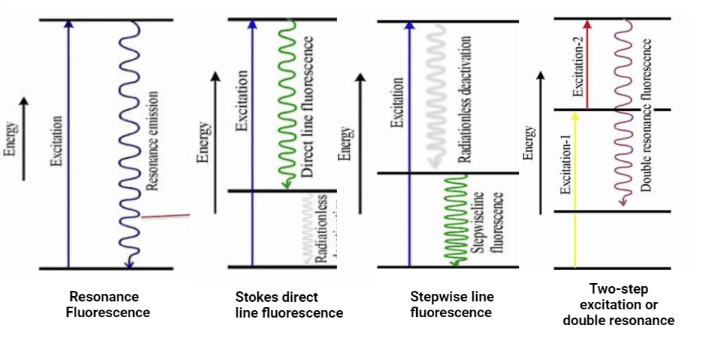
Types of Atomic Fluorescence
- Resonance fluorescence
- Stokes direct line fluorescence
- Stepwise line fluorescence
- Two-step excitation or double resonance
- Sensitized fluorescence
1. Resonance Fluorescence
When the excited states emit a spectral line of the same wavelength as that of excitation, resonance fluorescence occurs. It is mostly employed for analytical determination.
2. Stokes direct line fluorescence
It is observed when an atom that has been excited to higher energy by radiation absorption emits radiation and returns to a lower intermediate level. It lowers to the ground state by a radiation-less process from this intermediate level. As a result, the wavelength at which direct line fluorescence occurs will never be the same as the wavelength of the resonance line that excites it. It is also called Stokes fluorescence. Resonance fluorescence encounters interference from scattered radiation, which can be eliminated by utilizing direct line fluorescence.
3. Stepwise line fluorescence
In this kind of fluorescence, an atom that has been excited to a higher energy state by the absorption of radiation is deactivated to a lower excited state through a radiationless process, where it emits radiation to return to the ground state.
4. Two-step excitation or double resonance
Two dye lasers are used in a two-step excitation procedure for the double resonance fluorescence. The analyte is excited by the first laser from its ground state to an excited state, from which it is excited by the second laser to an even higher excited state. Fluorescence emission occurs along with the de-excitation of this more highly excited state to a lower energy state. This is called double resonance fluorescence.
5. Sensitized fluorescence
Sensitized fluorescence occurs when an excited atom transfers its energy to another atom, which is then excited and relaxes again while emitting fluorescence. The following diagram illustrates how sensitized fluorescence emission occurs.
S# + A → S + A#
A# → A + hγ ( Fluorescence)
However, sensitized fluorescence is generally not employed for analytical purposes.
Instrumentation of Atomic Fluorescence Spectroscopy
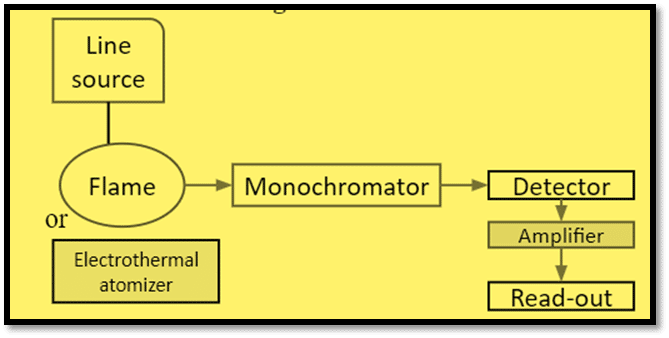
The basic components of atomic fluorescence spectroscopy are explained below:
- Line Source: Hollow cathode lamps (HCL), Electrodeless discharge lamps, and Lasers are generally employed as the line source.
- Nebulizer-burner system: The production of gaseous atoms is the primary objective of the nebulizer-burner system. The flame used in the AFS must be able to evaporate the liquid droplets and decompose the solid compounds leading to the formation of the gaseous atom. The flame source is made up of a variety of parts, including a pressure regulator, a flow meter for fuel and oxidant, an atomizer, and a burner. The different fuel gas used are acetylene, hydrogen, propane, etc. Air, oxygen, and nitrous oxide are the oxidants employed.
- Monochromators/Filters: Monochromators are used for the isolation of flame emission lines or bands to pass through them.
- Detector, Amplifier, Readout: The detector detects the emitted light and converts it into an electrical signal, amplified by the amplifier, and the data displayed by the read-out device.
Interferences in AFS
Due to the inclusion of additional materials in the sample, the radiation intensity might not precisely reflect the concentration of the sample. These materials interfere with the analytical process. Some of the common interferences observed in fluorescence spectroscopy are:
- Spectral interferences
- Chemical Interferences
- Ionization interferences
- Cation – anion interferences
- Cation – cation interferences
- Oxide formation interferences
Advantages of Atomic Fluorescence Spectroscopy
- It is a relatively simple and more sensitive technique than atomic absorption spectroscopy.
- Precision up to 1% can be achieved.
- More sensitivity even at low concentrations.
- Without the use of chemical separation methods, two substances that are stimulated at the same wavelength but emit at distinct wavelengths can be easily distinguished.
Disadvantages of Atomic Fluorescence Spectroscopy
- It has the drawback of quenching and background noise brought on by radiation dispersion by flame particulate matter, especially with refractory samples in high-temperature flame.
- Not applicable for all compounds due to fluorescence issues.
- Not ideal for identification.
Applications of Atomic Fluorescence Spectroscopy
Some of the major applications of Atomic Fluorescence Spectroscopy are:
- More than sixty distinct elements contained in a wide range of samples have been analyzed using atomic fluorescence spectrometry. AFS is typically used to determine the concentration of elements in samples.
- In the agricultural field for the analysis of dairy, wine, feed, meat, cigarettes, and other products for As, Hg, Pb, Sb, Se.
- Analysis of Pb, Hg, As, Sb, Bi, Ge, Se, in blood, urine, tissue, nail, and hair; Cu, Zn and Pb in blood serum and urine samples.
- Analysis of ore, rock, mineral, and metals for Ge, Hg, Se, As in Sb, Se, Te in Cu.
- For the determination of sulphide ion in sewage waters.
- In petrochemical for the quantitative determination of Pb, Hg, Cd, As, Sn, Zn in fuels, lubricant, and crude oil.
- Determination of Hg, Pb, As, and Se in active ingredients and fillers (Pharmaceutical industry).
Atomic Fluorescence Spectroscopy Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- D.A. Skoog, F.J. Holler, and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.






