Table of Contents
ToggleNon Aqueous Titration deals with the analysis in a medium completely free from water. It is the titration free from water. In other words, It is the titration of substances dissolved in solvents other than water. Non aqueous titration is carried out for the quantitative determination of medicinal drugs.
What is non aqueous titration?
Non-aqueous titration is an acid-base titration involving solvents other than water i.e there is no involvement of water. Non-aqueous titrations are the titrations in which weakly acidic or basic substances are estimated using non-aqueous solvents to get a sharp end point.
Why is non-aqueous titration carried out? Generally, water is used as a solvent for various titrations but non-aqueous titration is carried out:
- When reactants are insoluble in water.
- When reactants are reactive with water.
- When reactants are very weak acid/base in nature where 100% dissociation can not be achieved.
Theory of Non Aqueous titration
This titration is mainly carried out for weak acids and weak basic substances. When titration is carried out in an aqueous medium, the water due to its amphoteric nature acts as a strong acid. Thus, actual titration takes place between the strong acid( water) and weak base, so the exact endpoint can not be determined. Therefore, the importance of titration without water arises.
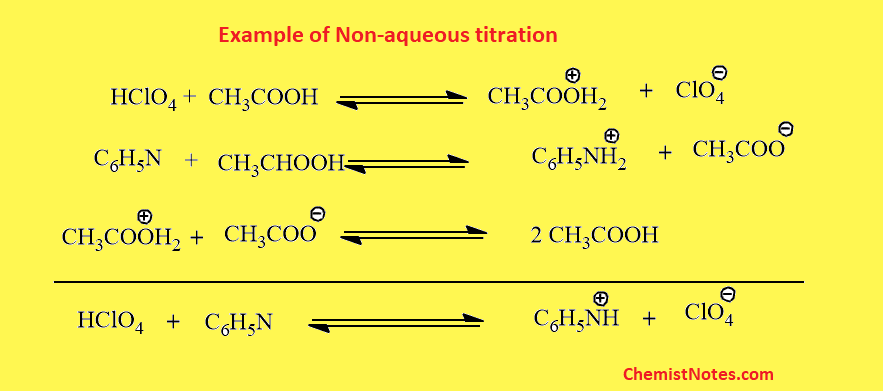
Example: Determination of pyridine
Since pyridine is a very weak base, it can be determined by this titration. Pyridine is dissolved in acetic acid, then titrated with perchloric acid dissolved in acetic acid.
Types of non aqueous titration
There are two types of nonaqueous titration.
- Acidimetry
- Alkalimetry
Acidimetry
Acidimetry is used for the quantitative estimation of weakly basic drugs. This is called acidimetry because the titrant used here is acidic in nature. For example; Perchloric acid. In addition to titrant, the protogenic solvent is used. Therefore, one of the known examples of acidimetry is perchloric acid in glacial acetic acid (protogenic solvents).
- Basic drugs such as Ephedrine, Morphine, Adrenaline, Caffeine, etc. can be determined by non-aqueous acidimetry.
- Generally, crystal violet (0.5% in acetic acid) is used as an indicator, which changes color from violet to light green at endpoints.
Alkalimetry
Alkalimetry is used for the quantitative estimation of weakly acidic drugs. This is called alkalimetry because the titrant used in this titration is basic in nature. For example, sodium methoxide, tetrabutylammonium hydroxide, etc are used as titrants. In addition to titrants, protophilic solvents such as DMF are used.
- Acidic drugs such as Acetazolamide, Fluorouracil, Allopurinol, Mercapto Purine, etc can be estimated by non-aqueous alkalimetry.
- Generally, Thymol blue(0.5% in methanol ) is used as an indicator, which changes color from pink to blue at the endpoint.
Titrants used in non aqueous titrations
The titrant is a solution of known concentrations that is titrated to another solution with an unknown concentration to determine the concentration. There are two types of titrants commonly used in such titrations.
- Acidic titrants: These are used in Acidimetry. Examples include Perchloric acid in dioxane, perchloric acid in glacial acetic acid, etc.
- Basic titrants: These are used in Alkalimetry. Examples include Quaternary ammonium bases such as Tetrabutylammonium hydroxide.
Indicators used in non aqueous titration
Various visual indicators have been found to be the most suitable for the detection of the endpoint in non-aqueous titration. These indicator changes the color or undergoes precipitation at the endpoint. Some indicators have been used and discussed below:
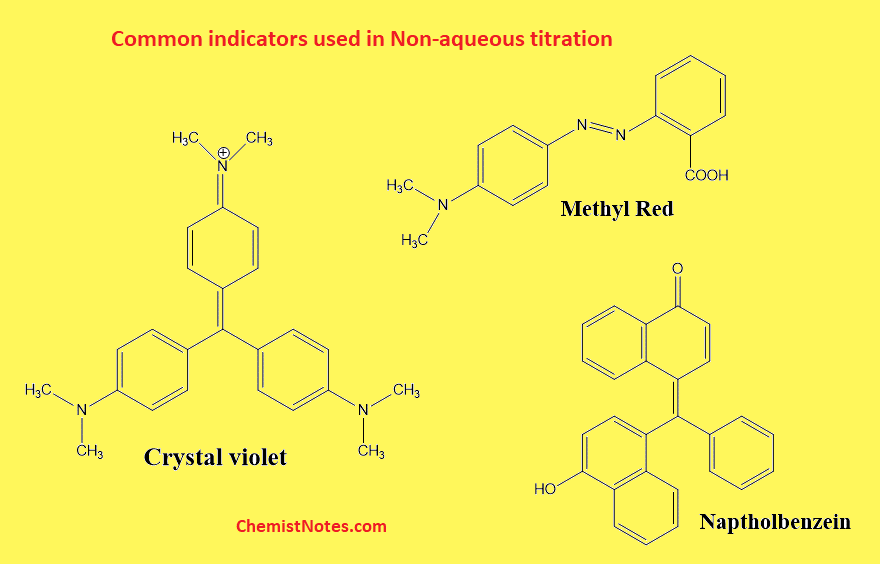
Crystal violet
It is a triarylmethane dye. It is prepared in 0.5% w/v solution in glacial acetic acid. Color changes from violet(basic) to light green (acidic). i.e. It gives a violet color in the basic medium and a light green color in the acidic medium.
Methyl Red
It is prepared in 0.2% solution in dioxane. The color change from yellow(basic) to red(acidic).
Naptholbenzein
It is prepared in 0.2% solution in glacial acetic acid. The color changes from yellow(basic) to green(acidic).
Solvents used in non aqueous titration
There are some important non-aqueous solvents and not all of them are suitable for titration. The following points should be considered during the selection of non-aqueous solvents.
- Solubility of analyte: Analytes should be soluble in the solvents.
- Nature of analyte: If the sample is acidic, the solvent can be basic and vice-versa.
- Reactivity of the analyte: The analyte should not react with solvent.
Types of solvent in Non Aqueous titration
- Protogenic solvent
- Protophilic solvent
- Amphoteric solvent
- Aprotic solvent
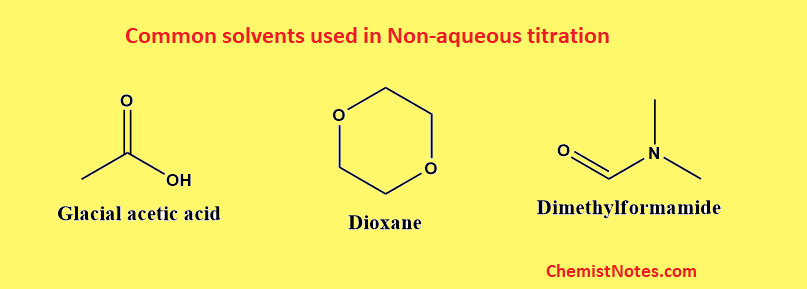
Protogenic solvent
These solvents are acidic in nature. Such solvents have a strong tendency to donate protons. These solvents are used to dissolve basic samples. They enhance the strength of weak bases. Examples of such solvents include glacial acetic acid, HF, and H2SO4.
Protophilic solvent
These solvents are basic in nature and have a very high affinity for the proton. These are used to dissolve acidic samples. Examples of such solvents include Liquid ammonia, pyridine, DMF, ethylenediamine, etc.
Amphoteric solvent
These solvents act as both weak acids and weak bases. Such solvents are able to accept as well as donate protons according to the chemical environment. Examples include Alcohol (methanol, ethanol,), and weak organic acids.
Aprotic solvent
These solvents are neither acidic nor basic in nature and don’t participate in the proton-accepting and donating process. These solvents don’t undergo a reaction with acids and bases and don’t cause ionization of samples thus, these are chemically inert solvents. These are mainly used to dilute the reaction medium and dissolve the water-insoluble drugs. The example includes Benzene, chloroform, and Carbon tetrachloride.
Application of non aqueous titration
It has various applications in numerous fields, especially in medicinal fields.
- It is used to know the purity of assays.
- It is used to determine the concentration expressions.
- It is used in the determination of hydrophobic compounds, diuretics, and steroids.
- It is used to determine the composition of antitubercular drugs and adrenergic drugs.
- It is used to quantify the mixtures of primary, secondary, and tertiary amines.
- It is used for studying sulphonamide, a mixture of purines, and many other organic amine compounds and salts of organic acid.
Advantages of non aqueous titration
- Organic acids and bases that are insoluble in water are soluble in a non-aqueous solvent and can be titrated by non-aqueous titration.
- Titration of weakly acidic and basic drugs gives a poor endpoint in aqueous titration but in non-aqueous titration, they give a sharp end point.
- It is used for the titration of water-sensitive drugs like aspirin.
- By the proper choice of the solvents or indicators, the biological ingredients of a substance whether acidic or basic can be selectively titrated.
- Non-aqueous titration is a simple, fast precise, and accurate method.
Disadvantages of non aqueous titration
- Temperature, moisture, and CO2 should be controlled otherwise error occurs.
- Solvents are expensive.
- Volatile solvents are toxic.
- Removal of water is necessary ( can take water from the air).
- Some solvents are hazardous if they are mishandled such as ammonia solution.
- Indicators must be prepared in a non-aqueous medium.






