Table of Contents
ToggleFactors affecting fluorescence, the introduction of fluorescence, fluorescence examples, the principle of fluorescences, types of fluorescence, and its applications have been discussed in this portion.
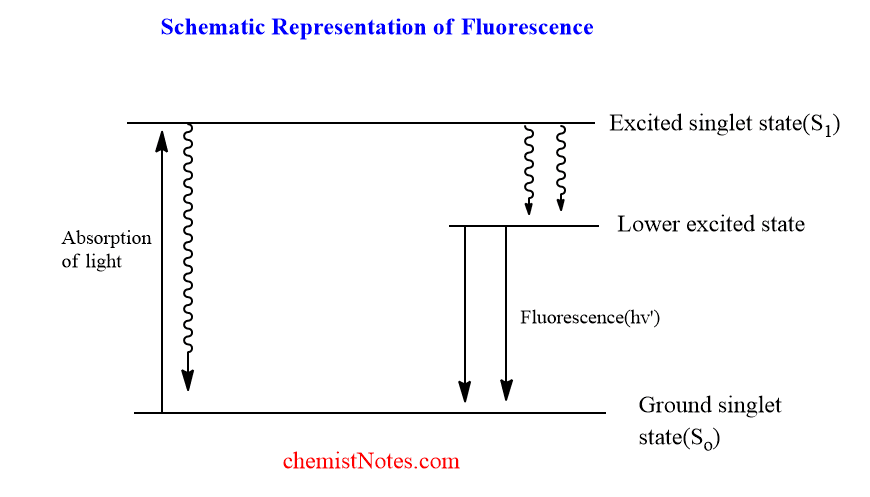
Fluorescence is a radiative relaxation process in which light is emitted as a result of the transition from S1 (excited singlet state) to So (ground singlet state) without change in spin multiplicity. This process can also be represented in the Jablonski diagram.
what is fluorescence?
When light is incident on certain substances, these substances emit visible light or radiations. Such a phenomenon is known as fluorescences. Fluorescence stops as soon as the incident light is cut off. Such types of materials which emit light when irradiated by light, are called fluorescent materials. This is the fluorescence definition.
fluorescence examples
There are some materials that emit light on irradiation with light. When light is incident on fluorite (CaF2), it emits blue light. Similarly, chlorophyll emits red light when dissolved in ether. Some examples of fluorescent substances are given as:
- Organic dyes like Fluorescein, and Eosin.
- Quinine sulfate emits blue light.
- Some glasses emit different colors when an x-ray falls on them.
principle of fluorescence
When light strikes a fluorescent substance, it absorbs the energy, causing atoms or molecules to be excited. However, if the light absorbed is insufficiently intense to eject electrons, electronic excitation occurs, in which the electron moves from the ground singlet state to the excited singlet state. Light of a different frequency is released when these excited electrons return to the ground state.
Radiation may be emitted by the excited atom or molecules in successive stages. The frequency of emitted radiations can be the same as or different from that of incident radiation.
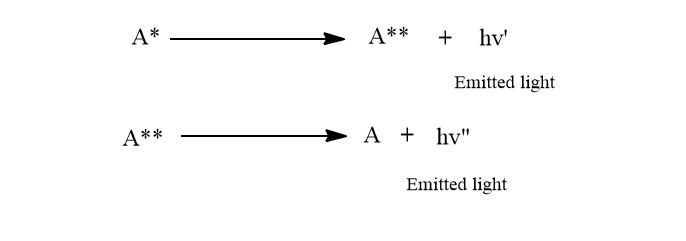
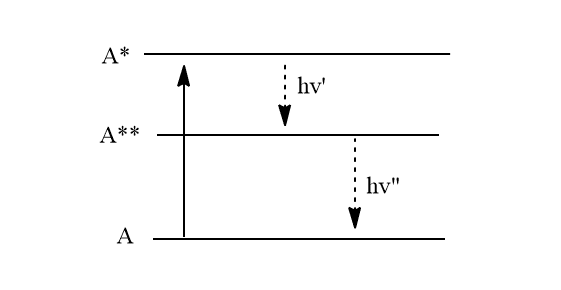
Fluorescence is an instantaneous phenomenon that begins as soon as the light is absorbed and ends as soon as the incident light is cut off. The absorbed energy may be released in 10-6 seconds or less, but in stages, resulting in radiations of varying frequency. Fluorescence is a secondary phenomenon that occurs as a result of the primary process of light absorption by an atom or molecules.
factors affecting fluorescence
Factors that affect the fluorescence are given below:
- Conjugated double bonds: Molecules with a greater extent of conjugated double bonds absorbs a greater amount of light, thus causing more intense luminescence.
- Effects of substituents: Generally electron-withdrawing groups such as -NO2, -COOH group, and -X(halide) decrease or sometimes destroy the fluorescence. Similarly, electron-donating groups such as -NH2, and -OH group increases fluorescence.
- Effect of pH: The fluorescence of chemicals is also influenced by pH. For example, a neutral or alkaline solution of aniline shows fluorescence in the visible region but in an acidic solution, visible fluorescence disappears.
Types of fluorescence
There are two types of fluorescence namely resonance fluorescence and sensitized fluorescence. Let’s explain these briefly.
Resonance fluorescence
It is well understood that when light falls on a fluorescent substance, it emits light. This process is called fluorescence. Is the frequency of emitted light exactly the same as the frequency of absorbed light? This is not always the case, but the frequency of emitted light may be different from that of absorbed light by the substance.
If the frequency of light absorbed by a fluorescent substance is exactly the same as the frequency of emitted light, then such a phenomenon is called resonance fluorescence.
Sensitized fluorescence
If two substances among which only one can exhibit fluorescence after irradiation with light, are taken together and irradiated then both may emit fluorescence simultaneously. This phenomenon is called sensitized fluorescence.
Therefore, a non-fluorescence substance can fluorescence if irradiated together with a fluorescent substance. In this case, the fluorescent substance absorbs light energy and then transfers to the non-fluorescent substance, which gets excited and emits light during coming back to the ground state.
fluorescence quenching
The intensity of fluorescence radiation may be reduced or prevented when a photochemically excited atom has a chance to collide with another atom or molecule before it fluoresces. This process is called quenching of fluorescence.
When an atom or molecule is photochemically excited, this excited atom has a chance to collide with another ground state atom of other chemical species. In such collision, energy is transferred to the atom of other species due to collision, and therefore, the excited atom returns to its ground state without emitting light radiation. This process is termed as Fluorescence quenching.
If only a fraction of the energy of the excited atom of fluorescent material is transferred to other non-fluorescent species, then the energy of fluorescent species is decreased. In such a case, fluorescence with reduced intensity is observed.
Application of Fluorescence
The main applications of fluorescence are shown as:
- In making fluorescence tubes
- In making different types of fluorescent dyes and paints.
- Many compounds exhibited fluorescent colors as a result of ultraviolet light absorption, such property that is used to test and identify the materials in the industry.
- In fluorescence microscope
- In television screen






