Table of Contents
ToggleQuadrupole mass analyzer
A quadrupole mass analyzer, also known as a quadrupole mass filter, is one of the most widely used analyzers. Its foundational element is the quadrupole mass filter, which Nobel Prize winner Wolfgang Paul and colleague Helmut Steinwedel initially described in 1953.
Why is it called a mass filter?
A quadrupole mass analyzer is considered a mass filter because of its ability to select ions of a particular m/z value for analysis, and ions are basically separated by applying both direct and alternating voltage. As the name implies, it is made up of four cylindrical rods that are parallel to one another, and the separation of the ions takes place on the basis of their ability to follow stable trajectories in the oscillating electric fields (a combination of both direct and alternating current) that are applied to the rods.
Principle of quadrupole mass analyzer
Quadrupole mass analyzers consist of four parallel cylindrical rods of a hyperbolic cross-section. The rods are charged by direct current and radiofrequency voltages to affect the movement of ions and are charged in such a way that two rods that are diagonally opposite to one another have the same voltage, while those that are perpendicular have a voltage with the opposite sign.
The ions must enter the quadrupolar field slowly (with only a few eV of kinetic energy) in order to interact with the vibrating electromagnetic field located between the rods. In direct current, voltage (U) is constant, and in alternating current (Vcosωt), the direction of voltage is changed. If an alternating voltage is applied, then the chance of hitting the metal rod depends on the mass and charge of the ion and also on the strength of the field and frequency of the oscillation. The strength of the field and frequency of the oscillation can be tuned by the operator.

If a high frequency is applied, then the ion simply has no time to react and nothing happens, but if the frequency is too low, then the ion is pulled into a rod before the potential flips. In the case of positive ions, the rod with positive potential would repel the ions, while the rod with negative potential would attract the ions and trap them.
If the DC field and the AC field are combined, then the positive ions are forced into the middle of the two positive rods while the AC field destabilizes them. If the ions have a high mass or low charge, then they are hardly affected by the AC field, and their trajectory is mostly determined by the DC field. The trajectory of the ions with too-small mass-to-charge ratio is however strongly destabilized by the AC field and causes them to crash into a rod. Electrodes with negative potential will attract the positive ions, and by applying suitable AC voltage, one can prevent the positive ions from hitting the rod.
Simply put, the potential of two opposing rods is positive except for a brief period of time when the negative RF voltage surpasses the positive DC voltage. Then, during this time, the rods will only defocus the lighter positively charged ions because the lighter ions have high velocity and therefore will strike on the rods and are not detected.
Additionally, this also means that these rods may be regarded as high-mass filters because they will only detect positively charged ions with a high mass-to-charge ratio. Similarly, the other two rods will focus only on the lighter ions for a short period of time when the RF voltage is positive on the rods, and thus they may be regarded as low-mass filters. If the voltages are properly selected, then a narrow mass-to-charge ratio range is capable of passing through the rods and interacting with the detector.
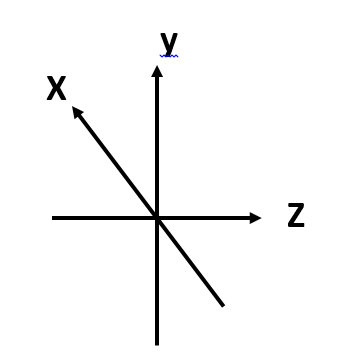
The stability of ions can be detected by simply calculating the forces that relate to the electric field in the quadrupolar field. The potential at any point in a hyperbolic field.
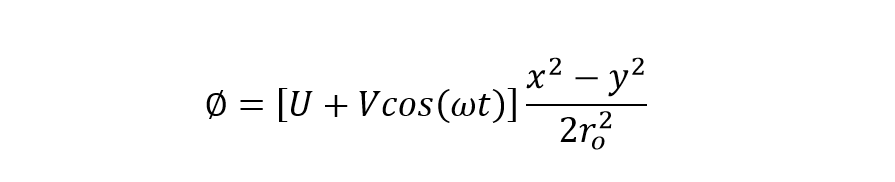
The electric field along each plane is the partial derivative with respect to the x, y or z-axis.
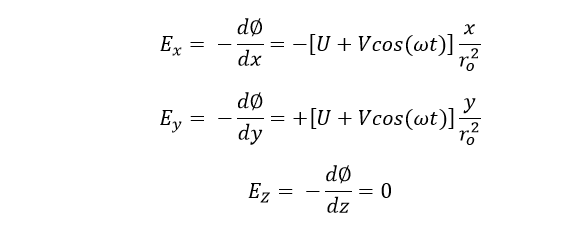
Where ω = angular frequency
ro = radius of quadrupole
According to Newton’s law,
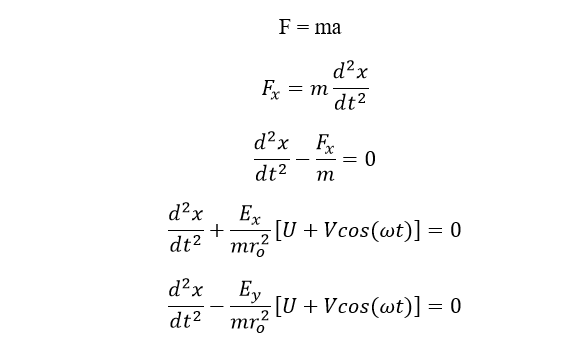
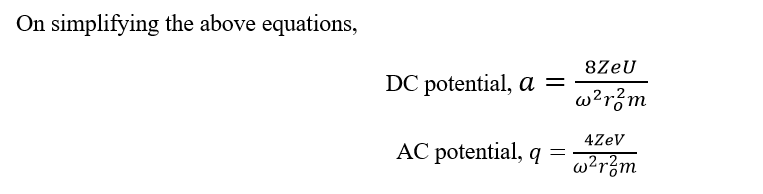
On plotting AC voltage vs DC voltage in a two-dimensional co-ordinate axis, we can find the stable trajectories as:
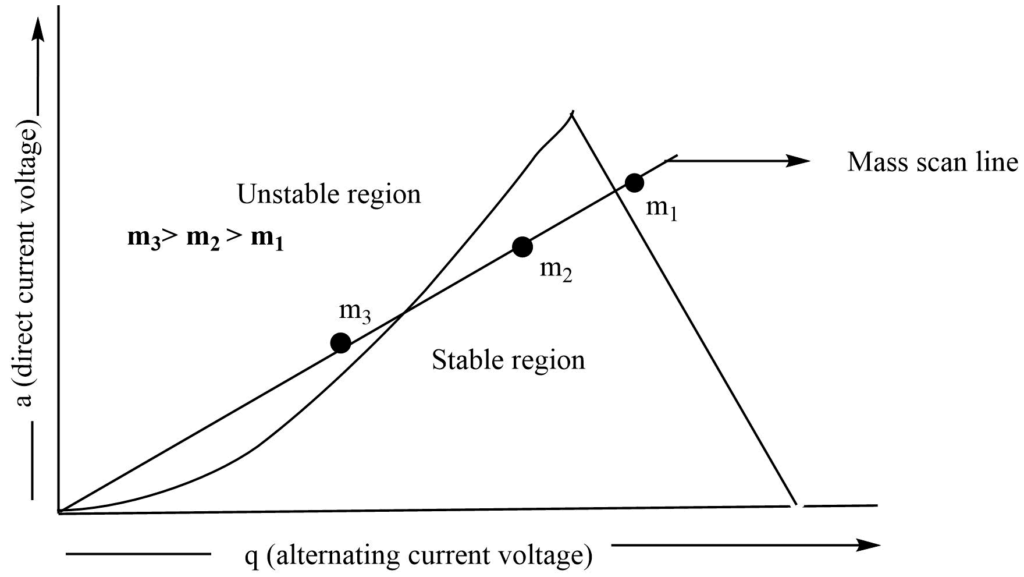
Figure: Plot of direct current voltage vs. alternate current voltage to determine the stability of ions
Working Principle of Quadrupole mass analyzer
A quadrupole mass analyzer consists of four parallel cylindrical metal rods arranged equally apart from the centre axis. In order to ensure that only the ions with the desired m/z successfully travel through the quadrupole and reach the detector, the quadrupole is subjected to both a direct current and a high-frequency alternating current, or radiofrequency. A signal is generated from the number of ions that enter the detector and is displayed on the computer.
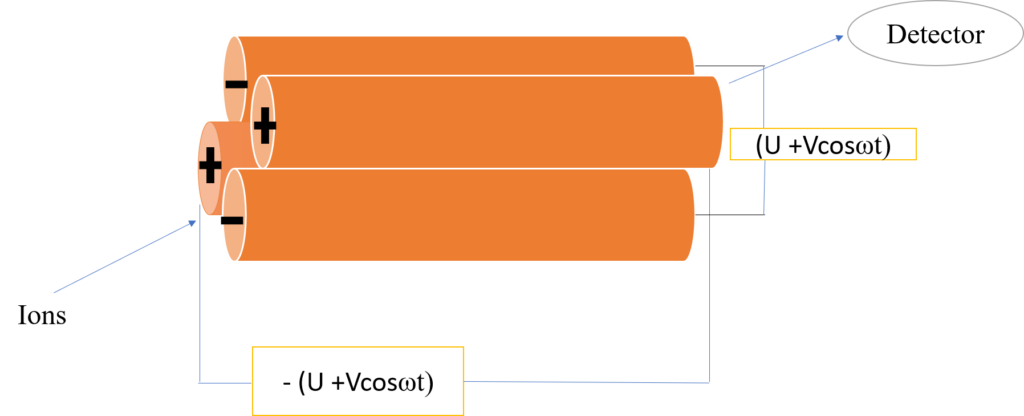
Figure: Schematic diagram of quadrupole mass analyzer
Firstly, ions are ionized using an ionization technique like electrospray ionization, chemical ionization, matrix-assisted laser desorption ionization (MALDI), and many more. Then, it is passed onto the quadrupole mass analyzer where ions with stable trajectories are detected in the detector, whereas ions with unstable trajectories are not detected. The possibilities of the ions being detected can be controlled by tuning the strength of the field and the frequency of the oscillation.
Initially, a rather small voltage of only a few hundred volts is used to accelerate the continuous ion source produced in the ionization unit in the z-direction. These ions enter the quadrupole through a small entrance. The voltage of the opposite polarity is applied to the neighbouring poles while the same polarity voltage is applied to diagonally opposite poles. The quadrupole produces an electric field with a rapidly shifting phase when alternating current and direct current voltage are supplied in conjunction with each pole.
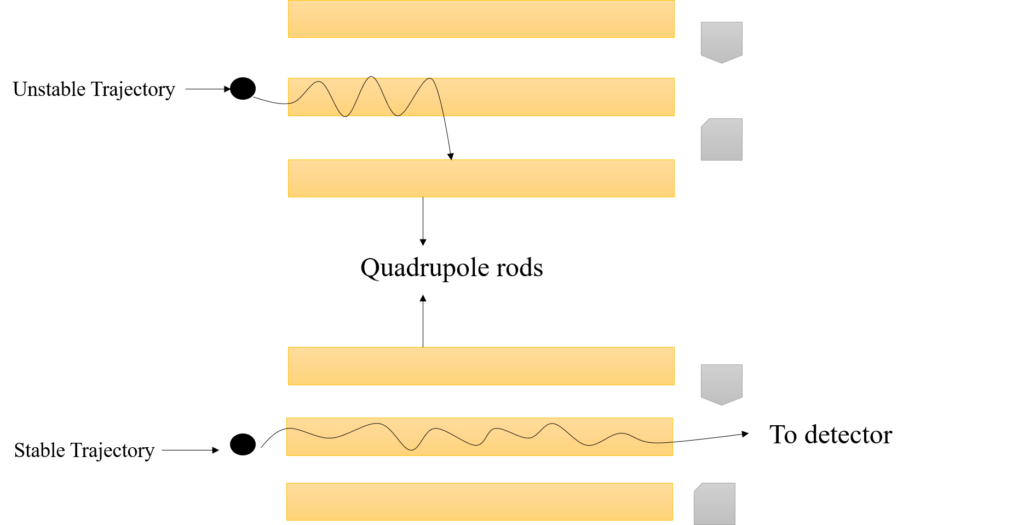
Figure: Schematic diagram of detected and non-detected ions in quadrupole mass analyzer
Ions moving through this electric field therefore fluctuate in the x- and y-directions, and ions with specific m/z possess a steady oscillation and pass through the quadrupole to reach the detector when a specific set of parameters is applied. The oscillations of the ions with different m/z values, on the other hand, become unstable, leading them to strike the rods, escape the system and therefore, be undetected.
How are gaseous ions separated in a Quadrupole mass analyzer?
Gaseous ions are separated in a quadrupole mass analyzer on the basis of the stability of pathways or trajectories in the oscillating electric fields that are applied to the rods. The application of the combined alternating current and the direct current to the rods will determine the trajectories. This electric field will be generated if the radiofrequency current is supplied to one of the opposing pairs of rods within the quadrupole. The chance of ions being detected can be increased by tuning the strength of the field and the frequency of the oscillation by the operator, and the frequency required for a stable oscillation can be determined from the equations.
How does quadrupole mass analyzer perform mass selection?
A quadrupole mass analyzer performs mass selection by varying the AC and DC voltages. Let us consider that if only, an alternating potential is applied, then in this case, when the potential is positive, positive ions will be repelled and will stay in the centre of the two rods. In the same way, when the potential is negative, positive ions will accelerate to the rods and will not be detected.
If the AC voltage and positive DC voltage are supplied at the same time to the pair of rods, ions of different masses will respond differently to the applied potential. Heavy ions are not affected by the alternating current and therefore, will be detected by the detector because it will be focused at the centre of rods as even under the application of RF voltage, it won’t be much affected because its velocity is much slower as compared to lighter ions and in this case, it will behave as a high mass filter.
Similarly, the other pair of rods will be subjected to the converse of AC/ DC potential and thus, it will have negative DC potential, and because it is negative, the heavy ions will strike the electrode and will not be detected but the lighter ions will respond to the AC potential and will be focused in the centre of the quadrupole. So, in this case, lighter ions will have a stable trajectory and will behave as a low-mass filter.
A single mass-to-charge ratio can be selected by combining both electrodes into the same system and allowing it to have a stable trajectory. The stable trajectories are also altered if the magnitude of the AC and the DC are altered. Different ions have different stable trajectories at different magnitudes and will reach the detector at different times. So, simply by varying the voltage of AC and DC, the desired mass range can be selected.
Advantages of quadrupole mass analyzer
- Relatively Cheap and dynamic
- Fast and simple operation for high-throughput analysis
- Doesn’t require a very high vacuum condition (> 10-7 )
- Good reproducibility and classical mass spectra
- Compact size, fast scan rate, high sensitivity for trace analysis
- The close proximity to the detector and tiny ion source allows for smaller benchtop instruments than a standard GC.
- Tunable mass resolution.
Disadvantages of quadrupole mass analyzer
- Low mass range (<4000 m/z)
- Low resolution (< 4000)
- Poor mass accuracy (>100 ppm)
- Low scanning speed
- MS/MS needs multiple analyzers
Applications of Quadrupole mass analyzer
- Most often used for targeted, quantitative applications
- Characterization of antibody-drug conjugates
- Used for determining the amounts of different gases in a mixture
- Used in liquid or gas chromatography, where they serve as detectors with extraordinarily high specificities.
- Resolution of low-mass isotopes
- Analysis of pesticide spiked into an orange extract.
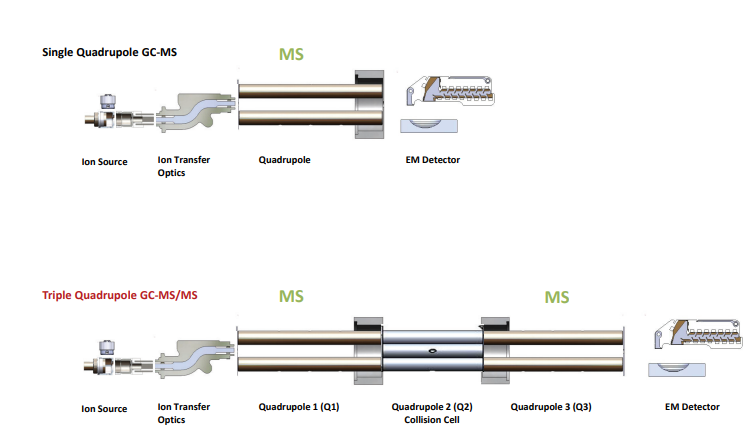
Single quadrupole mass analyzer
A single quadrupole mass spectrometer contains a single mass analyzer. The only ions that can be measured by single quadrupole mass spectrometers are those produced at the instrument source. These ions can be fragment ions or intact molecular ions produced by in-source fragmentation. Since, this type of analyzer is less specific than tandem quadrupole and also, does not offer as much structural information, a triple quadrupole mass analyzer is used. Also, it cannot detect variation in the signal generated by the analyte and the signal generated from the matrix.
Triple quadrupole mass analyzer
Triple quadrupole mass analyzers, also known as tandem quadrupole mass analyzers, consist of two quadrupole mass analyzers separated by collision cells. The first quadrupole mass analyzer chooses the precursor ions.
Then, the selected precursor ions are broken apart or fragmented in the collision cell by the process known as collision-induced dissociation (CID). Here, the analyte of interest collides with an inert gas, such as nitrogen or argon. The specific product ions generated by CID depend on the collision gas and the energy employed, as well as the bond energies inherent in the precursor ion’s molecular structure. If the CID conditions are consistent and reliable, product-ion patterns and relative ion abundance can be quite repeatable.
Source: https://www.researchgate.net/publication/349366813
Finally, the product ions are examined or selected by the second or final quadrupole mass analyzer, and then they are detected in the detector.
Advantages of Triple quadrupole mass analyzers
- Highly specific because, when the electric fields and the collision energy are held constant, only analyte ions with a certain mass transition (Precursor/ product ion pair) are able to reach the detector.
- Perfect for quantification of the target compound
- Works as a structure-selective detector, which means it has the ability to select the analyte out of an intense matrix.
- Rapid electrical control of quadrupoles leads to the detection of many hundreds of analytes or compounds in a single run.
Comparison of TOF and quadrupole mass analyzer
The major differences between TOF and quadrupole mass analyzers are tabulated below.
| TOF (Time of flight) mass analyzer | Quadrupole mass analyzers |
| It is based on the time the ions take to reach the detector through the flight tube. | It is based on the stability of trajectories in the oscillating electric fields that are applied to the rods |
| The velocities of the two ions differ depending on the mass of the ions, although the ions have the same kinetic energy. Smaller ions have high velocity and therefore, are detected first. | The chances of ions being detected can be controlled by tuning the strength of the field and frequency of the oscillation |
| Unlimited mass range and measures all m/z at once. | Low mass range and measures each m/z separately |
| Can accumulate full ten seconds of signal for each m/z | Can only spend one second for the accumulation of signals for each m/z |
Published by: Soniya Joshi
Quadrupole analyzer video
References:
- Mellon, F. A. MASS SPECTROMETRY | Principles and Instrumentation. In Encyclopedia of Food Sciences and Nutrition (Second Edition); Caballero, B., Ed.; Academic Press: Oxford, 2003; pp 3739–3749. https://doi.org/10.1016/B0-12-227055-X/00746-X.
- Sreekumar, J.; Hogan, T. J.; Taylor, S.; Turner, P.; Knott, C. A Quadrupole Mass Spectrometer for Resolution of Low Mass Isotopes. J. Am. Soc. Mass Spectrom. 2010, 21 (8), 1364–1370. https://doi.org/10.1016/j.jasms.2010.03.041.
- Leary, J. J.; Schmidt, R. L. Quadrupole Mass Spectrometers: An Intuitive Look at the Math. J. Chem. Educ. 1996, 73 (12), 1142. https://doi.org/10.1021/ed073p1142.
- Dunn, W. B. Chapter Two – Mass Spectrometry in Systems Biology: An Introduction. In Methods in Enzymology; Jameson, D., Verma, M., Westerhoff, H. V., Eds.; Methods in Systems Biology; Academic Press, 2011; Vol. 500, pp 15–35. https://doi.org/10.1016/B978-0-12-385118-5.00002-5.
- Somogyi, Á. Chapter 6 – Mass Spectrometry Instrumentation and Techniques. In Medical Applications of Mass Spectrometry; Vékey, K., Telekes, A., Vertes, A., Eds.; Elsevier: Amsterdam, 2008; pp 93–140. https://doi.org/10.1016/B978-044451980-1.50008-2.






