Table of Contents
ToggleAmong the various separation techniques available at an analytical scale, reverse phase chromatography is the most favored and widely used method. This technique consists of a polar mobile phase and a non-polar(less polar) stationary phase. This versatile technique finds application across a broad range of molecules including charged as well as polar molecules. In this technique, variables such as organic solvent type, concentration, pH, and temperature can be controlled more precisely, thus making it a robust technique.
Principle of Reverse phase chromatography
We know, the organic molecule contains not only functional groups but also non-polar groups. The water in the mobile phase repels the non-polar regions of the solute molecules which facilitates their interaction with the non-polar functional groups of the stationary phase (Non-polar). This phenomenon is responsible for the retention in reverse-phase chromatography. Consequently, the solute molecules are eluted in a sequence based on their polarity. This implies that more polar compounds are eluted initially, followed by less polar and non-polar compounds.
If the analyte molecule is more non-polar (molar hydrophobic), it will spend more time on the non-polar stationary phase. Thus, for desorption, a higher concentration of organic solvent is required.
Commonly used stationary phases in Reverse phase chromatography
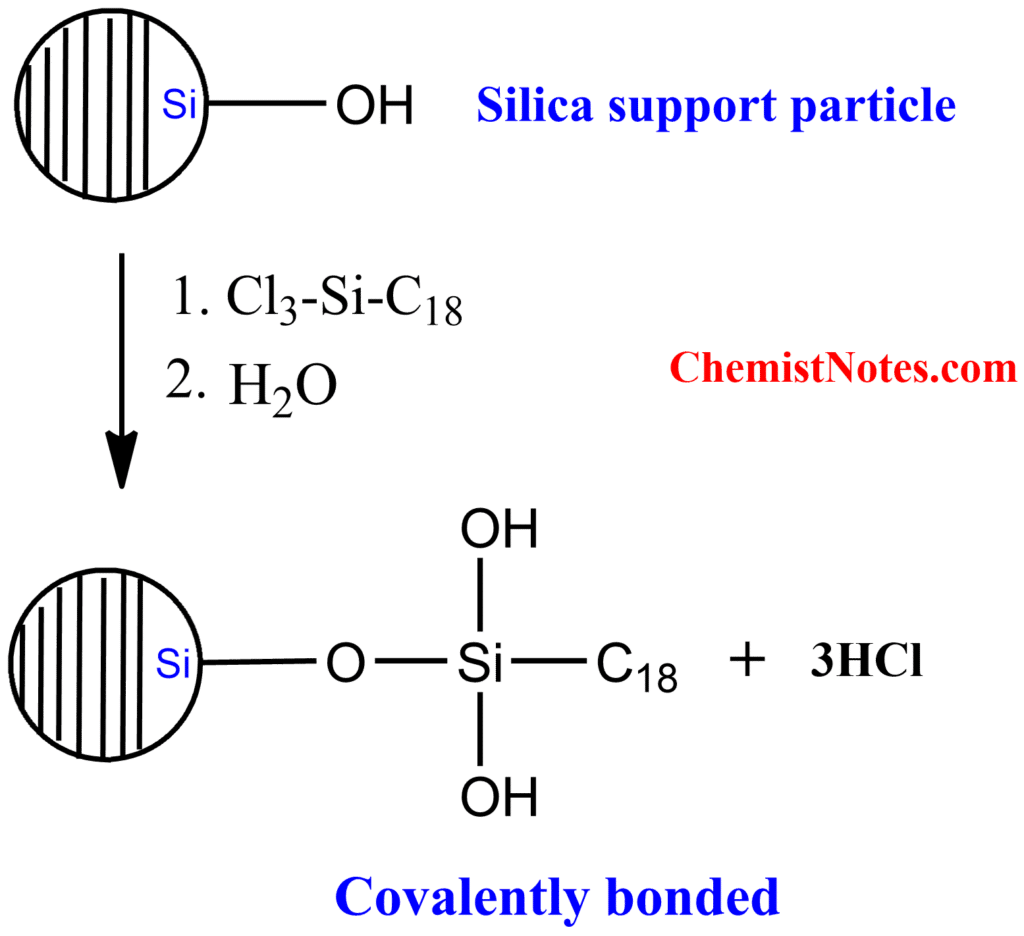
The widely used material for column packing is octadecyl silyl silica (ODS-C18) wherein silica is chemically modified with a C18 functional group. This is popularly known as the C18 column. Moreover, octyl(C8), hexyl(C6), propyl(C3), ethyl (C2), phenyl, and cyclohexyl functional groups are bonded to a silica surface which makes the silica stationary phase nonpolar and hydrophobic. The synthesis of reversed-phase packing material occurs as follows:
Commonly used mobile phases in Reverse phase chromatography
In reverse-phase chromatography, the mobile phase is more polar than the stationary phase. Typically, in reverse phase comprises water and water-miscible organic solvents like methanol, acetonitrile, and tetrahydrofuran.
Normal phase vs Reverse phase column
The major difference between the normal phase column and the reverse phase column is that the normal phase column consists of a polar stationary phase and a non-polar mobile phase while the reverse phase column consists of a non-polar stationary phase and a polar mobile phase.
Limitations of Reverse phase column chromatography
This is generally unsuitable for protein purification due to the presence of organic solvents, which can cause the denaturation of proteins and destroys their biological activity. Moreover, this technique requires more analysis time for complex mixtures.