Table of Contents
ToggleThe Time of Flight mass analyzer (TOF) is one of the most widely used mass analyzers in the mass spectrometric field. Similar to other mass analyzers, its main function is to separate charged particles formed by the ionization process. In this blog, you will learn about the development, principle, working mechanism, advantages, limitations, and applications of the Time of Flight (TOF) mass analyzer.
Time of flight mass analyzer (TOF)
The development of the Time of Flight mass analyzer (TOF) began in the late 1940’s. The first TOF equipment was created by A.E. Cameron and D.F. Eggers with a mass spectrum monitor in 1948 A.D.
Since this was the first TOF instrument, its resolution was low and the mercury ions were hardly detected, but now it has been extensively used due to the current advancements, including, reflectron, high-speed electronics, and orthogonal acceleration, leading to improved resolution.
Basically, the crucial characteristic that has made TOF-MS popular is the integration of
- A mass range that is theoretically not restricted to huge masses
- Extremely compatible with other selection procedures, and a design like Q-TOF, MALDI-TOF, and many more
- Simple work process
- Higher resolution
Time of flight mass analyzer principle
The principle of a Time-of-flight mass analyzer relies on the simple idea that ions with varying mass-to-charge ratios and the same kinetic energy move at different speeds in an electric field, which will determine the composition or structure of substances. It depends on the concept that ions are separated based on the time it takes for the ions to travel through a flight tube with a known length up to the detector. It is a kind of scanning technique where a full mass spectrum is acquired as a snapshot instead of sequentially stepping through a series of m/z values while acquiring the data.
Simply put, ions are accelerated through a potential (V) and allowed to drift down a tube to a detector. Since all ions have the same energy. So, the potential energy will be equal to the kinetic energy; thus, we get the mass-to-charge ratio relationship with that of velocity by solving it. As the mass-to-charge ratio is inversely proportional to the square of velocity, the higher the mass-to-charge ratio, the lower the velocity, which means low-mass compounds will have a greater velocity and will hit the detector first. Its mathematical demonstration is shown as:
Potential energy = Kinetic energy
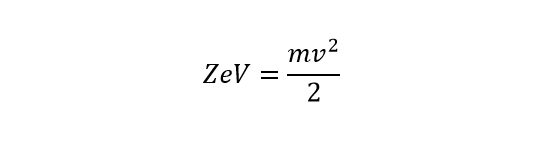
On rearranging,
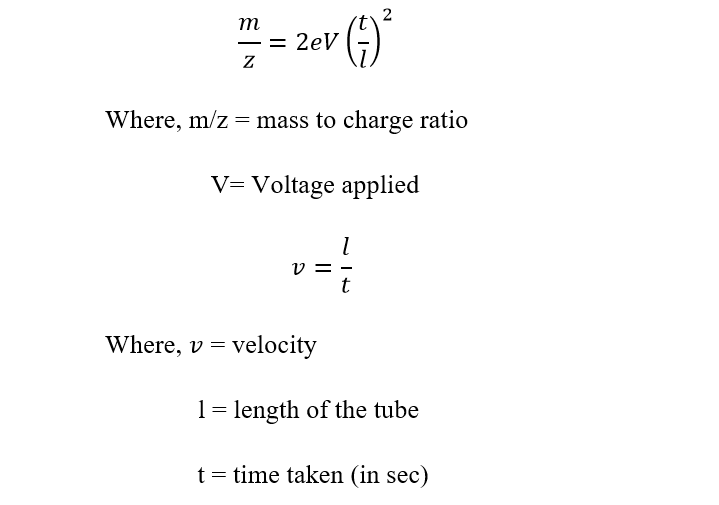
The flight time for each mass-to-charge ratio is different, starting with the use of a high-voltage pulse at the back plate of the ion pulse and ending after the ions hit the detector. The flight time can be easily calculated by taking the energy at which ions get accelerated, the mass-to-charge ratio, and the distance traveled by the ions and applying them to the above equation. The squared relationship between the mass and the flight time shows that if the observed time of the ion is tripled, the resulting mass is not tripled, but instead, it is nine times greater.
How does a time of flight mass analyzer work?
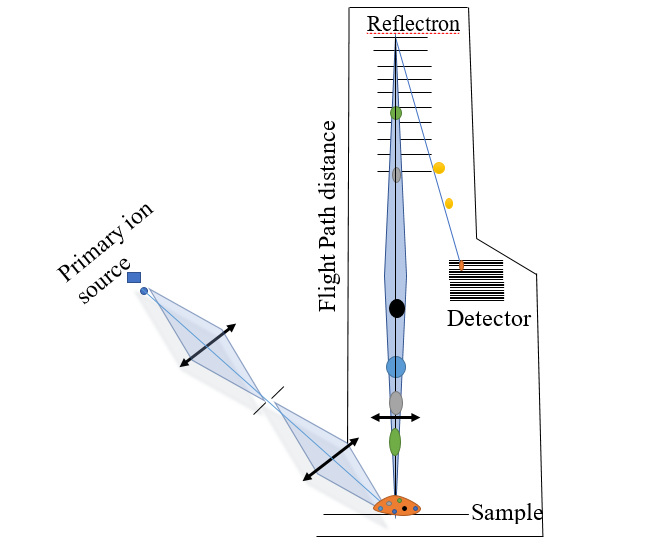
Figure: Schematic diagram of basic working principle of Time of flight mass spectrometry
The working mechanism of the Time of Flight mass spectrometer begins by ionizing the sample with a high-voltage electric pulse and accelerating the ions to the same potential. The ions move to the detector through a flight tube, and the signal is generated on the basis of the time it takes to reach the detector. Since all ions are subjected to the same potential, their velocity is solely dependent on their mass-to-charge ratio, and therefore, the smaller compounds reach the detector first.
This seems kind of simple, but in reality, there is a lag time between the start pulse transmitted by the control electronics and the generation of high voltage on the back of the ion pulser plate. There is also a gap from the time the ion hits the front surface of the detector to the signal generation in the computer. These lag times are short but play a crucial role. In order to evaluate the proper true flight time, the lagging time at the beginning and at the end needs to be subtracted by referring to it as to.
Therefore, the time taken is given by,
t = tm-to
Where tm = measured time taken by ions to travel to the detector through the flight tube
to = Sum of lagging time at the beginning and at the end of ion generation
All dynamic instruments can be referred to as “time of flight” according to the fundamental principle underlying ion separation, which is to use differences in the time taken to pass a certain path in the instrument. These instruments include simple time-of-flight mass spectrometers and TOF instruments with reflectrons.
In a simple TOF mass spectrometer, the resolution is not very good due to focusing errors in sources of all types caused by an ion energy spread or other factors, such as the limited thickness of the ion production zone. Resolution is impacted by the varying ions beginning times and positions before they are accelerated into the flight tube. Additionally, the improper findings are produced by low mass resolution caused by variable ion kinetic energy and beginning ion orientations.
What is the role of Reflectron?
Reflectron mainly corrects the poor resolution and improves mass accuracy. There is applied potential in the reflectron in which ions will be reflected in the opposite direction to the detector. Prior to the reflectron, the ions were spaced similarly, but after the reflectron, the ions were farther apart, leading to improved mass resolution. It simply minimizes the spread of energy among ions with the same mass-to-charge ratio.
The development of TOF-MS with reflectron was proposed by Russian scientist S.G Alikhanov. TOF-MS with Reflectron simply consists of an additional ion reflector, along with a field-free drift area and an ion source. Ions are accelerated in the ion source, travel through the field-free drift zone, are reflected in the ion reflector, travel through a portion of the field-free drift region once again, and then strike the surface of the ion detector as shown in the above figure.
Advantages of Time of Flight (TOF) mass analyzer
- High sensitivity with full scan
- Unlimited mass range
- Helps in analyzing a wide molecular weight range, including both polar and non-polar compounds
- Faster measurement of sample
- No loss of charged ions
- Capable of up to 500 spectra per second collection speeds regardless of mass range
Disadvantages of TOF-MS
- Low resolution (< 20,000)
- The difference in arrival times at the detector can be less than 10-7, so fast electronics are necessary for adequate resolution
Applications of TOF-MS
- Measurement of a mixture of Oligosaccharides
- Elucidation of the complete range of diclofenac phototransformation products resulting in the determination of seven polar transformation products
- Sequencing of proteins, peptides
- DNA sequencing instead of using gel electrophoresis
- Measurement of immune globulin IgG, bovine serum insulin
- Used for the drug screening of biological samples
TOF mass analyzer video
Published by: Soniya Joshi
References
- Silverstein, Robert M., and G. Clayton Bassler. “Spectrometric Identification of Organic Compounds.” Journal of Chemical Education 39, no. 11 (November 1962): 546. https://doi.org/10.1021/ed039p546
- Agüera, A., L. A. Pérez Estrada, I. Ferrer, E. M. Thurman, S. Malato, and A. R. Fernández-Alba. “Application of Time-of-Flight Mass Spectrometry to the Analysis of Phototransformation Products of Diclofenac in Water under Natural Sunlight.” Journal of Mass Spectrometry 40, no. 7 (July 2005): 908–15. https://doi.org/10.1002/jms.867
- Boesl, Ulrich. “Time-of-Flight Mass Spectrometry: Introduction to the Basics: TIME-OF-FLIGHT MASS SPECTROMETRY.” Mass Spectrometry Reviews 36, no. 1 (January 2017): 86–109. https://doi.org/10.1002/mas.21520.
- Dridi, B. B.; Drancourt, M. 13 – Characterization of Prokaryotes Using MALDI-TOF Mass Spectrometry. In Methods in Microbiology; Rainey, F., Oren, A., Eds.; Taxonomy of Prokaryotes; Academic Press, 2011; Vol. 38, pp 283–297. https://doi.org/10.1016/B978-0-12-387730-7.00013-9
- Gergov, M. Chapter 14 Forensic Screening with Liquid Chromatography-Mass Spectrometry. In Handbook of Analytical Separations; Bogusz, M. J., Ed.; Forensic Science; Elsevier Science B.V., 2008; Vol. 6, pp 491–511. https://doi.org/10.1016/S1567-7192(06)06014-1.
- Mamyrin, B. A. Time-of-Flight Mass Spectrometry (Concepts, Achievements, and Prospects). International Journal of Mass Spectrometry 2001, 206 (3), 251–266. https://doi.org/10.1016/S1387-3806(00)00392-4.






