Table of Contents
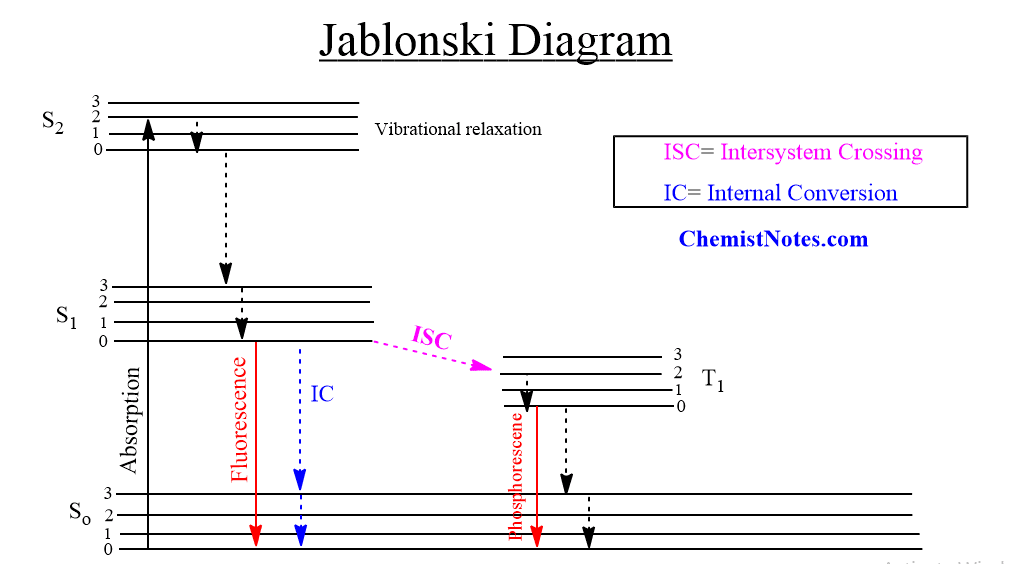
ToggleJablonski diagram is a graphical representation of the various transitions that can occur after a molecule has been excited photochemically. When a molecule is raised from its ground state to a higher state using light, photochemistry occurs. The molecule in the excited state has a shorter lifetime and significantly more energy than the ground state from which it was formed. As a result, molecules in the excited state are much more reactive. A photochemical or photophysical process deactivates an excited state. Therefore, the fate of the excited molecules is described by using the Jablonski diagram, which only focuses on the photophysical process occurring during the excitation and deactivation process.
Jablonski diagram explanation
When a molecule is photochemically promoted to an excited state, it does not stay there for long. The majority of promotions are from the So to the S1 state. Promotions from So to triplet are not permitted. Promotion to S2 and higher singlet states occurs. The energy lost when an S2 or S3 molecule degrades to S1 is given up to the environment in small increments. This process is known as the energy cascade.
Many of the vibrational levels of S1 are initially populated by the initial excitation and decay from higher singlet states, but these also cascade down to the lowest vibrational level of S1. Therefore, the lowest vibrational level of the S1 state is the only important excited singlet state. This state can go through a variety of physical and chemical processes, but only physical pathways are illustrated in the Jablonski diagram.
The excited state molecules undergo the following major physical processes.
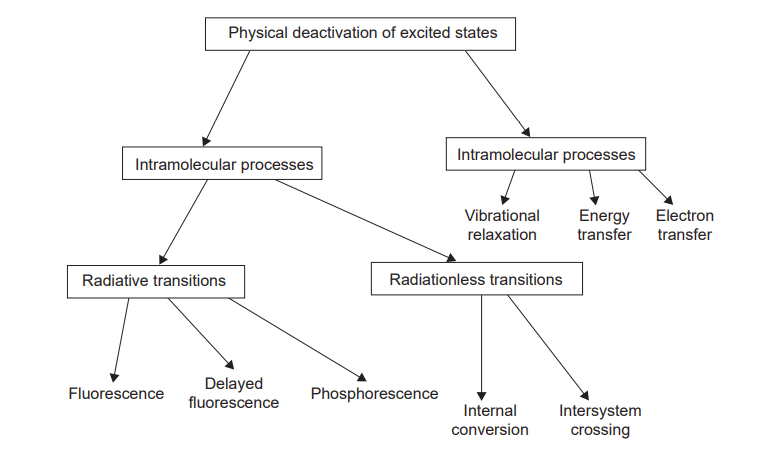
Internal conversion
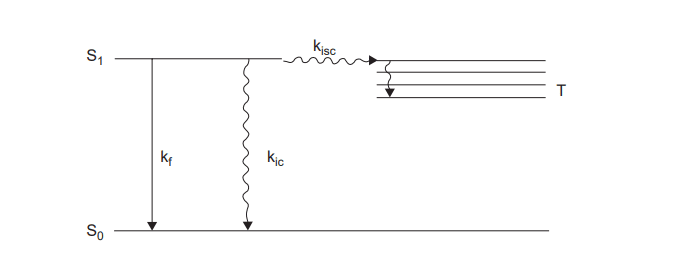
A molecule in the S1 state can cascade down through the vibrational levels of the So state and thus return to the ground state by releasing energy to the environment in small increments. This process is known as an internal conversion, and it is quite slow due to the large amount of energy involved.
Fluorescence
By releasing energy in the form of light, a molecule in the S1 state can instantly fall to a low vibrational level of the So state. Fluorescence is the name for this phenomenon, which occurs within 10-9 seconds.
Intersystem crossing
The majority of molecules in the S1 state may switch between electronic states with varying spin multiplicities i. e the lowest triplet state T1. This process is called intersystem crossing. There is no energy loss as a result of intersystem crossing. Because a single state usually has more energy than the corresponding triplet, energy must be sacrificed. As a result, T1 states at higher vibrational levels cascade down to lower vibrational levels.
Phosphorescence
A molecule in the T1 state can return to the So state by releasing heat via intersystem crossing or light. If the light is emitted, the process is known as phosphorescence. T1 states have significantly longer lifetimes than S1 states. Because of the greater energy difference between S1 and So than between T1 and So, phosphorescence is found at lower frequencies than fluorescence when they occur in the same molecules.
Photosensitization
A molecule in an excited state (S1 or T1) may transfer all of its surplus energy to another molecule in the environment at once if nothing else happens to it first. This process is known as photosensitization.
The excited molecule A thus drops to So while another molecule becomes excited. Let’s see an example:
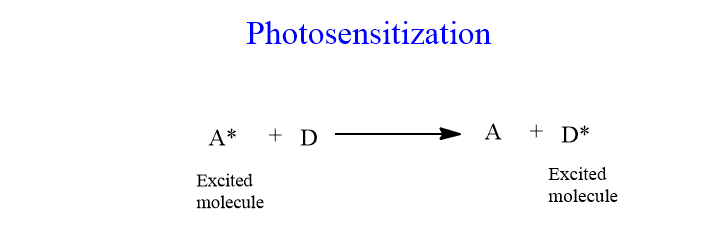
As a result, there are two methods for a molecule to get excited: absorption of a photon of light or transfer from a previously excited molecule. Donor A is also known as a photosensitizer.
Quenching
Quenching is a non-radiative process that involves the deactivation of an excited molecular entity by an external environmental effect or via a substituent. Therefore, an excited species can be quenched.
References
- Charles DePuy and Orville L. Chapman, Molecular Reaction and Photochemistry,
Prentice-Hall, 1972.






