Table of Contents
ToggleColumn chromatography can be categorized into normal phase column chromatography and reverse phase column chromatography based on the polarity of the stationary phase and mobile phase. Let’s discuss it briefly.
Normal phase column chromatography
Normal phase column chromatography is a robust technique for separating substances depending on their polarity by employing a polar stationary phase and a less polar mobile phase. It is an invaluable tool in the world of chemistry for separating and purifying components from complicated mixtures.
Principle of Normal phase column chromatography
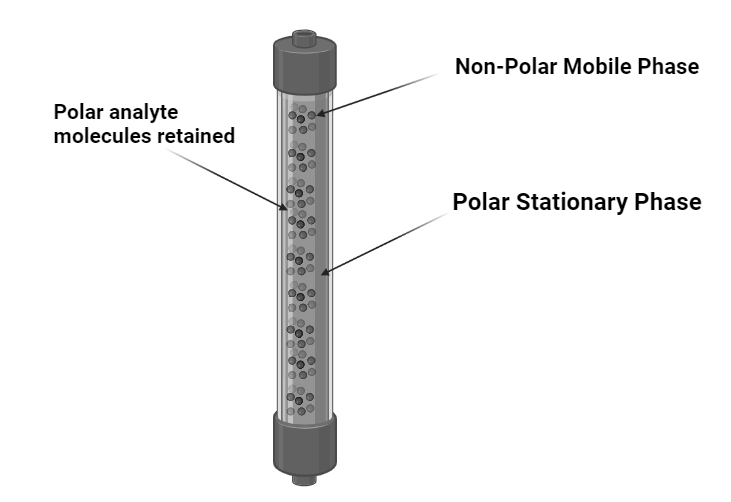
In Normal phase column chromatography, the stationary phase consists of polar substances while the mobile phase consists of nonpolar organic solvents. The separation of molecules in normal phase column chromatography is based on the variations in their affinity or interaction strength with the polar stationary phases.
The stationary phase in normal-phase chromatography is hydrophilic, meaning it has a high attraction towards hydrophilic molecules present in the mobile phase. As a result, hydrophilic molecules present in the mobile phase have a tendency to bind or be adhered to by the column, while hydrophobic molecules pass through the column and are eluted first. To elute hydrophilic molecules from the column, the polarity of the mobile phase solution can be increased.
Commonly used stationary phase in Normal phase chromatography
To achieve desired separations, it is crucial to choose the appropriate stationary phase and the eluent system carefully. In normal-phase chromatography, the stationary phase is typically composed of either inorganic adsorbents or chemically bonded phases on silica gel with modified polarity.
- Inorganic adsorbents: Silica, Alumina, etc.
- Modified phases: These consist of moderately polar chemically bonded phases which include functional groups like aminopropyl, cyanopropyl, nitrophenyl, and diol. These functional groups are chemically bonded to the silica gel support.
In normal-phase chromatography, the stationary phase is more polar compared to the mobile phase. Consequently, as the relative polarity of the stationary phase increases, and the polarity of the mobile phase decreases, the retention of the analyte is enhanced. Moreover, retention not only rises with increases in the polarity of the stationary phase but also with the number of adsorption sites present in the column.
Commonly used mobile phase in Normal phase column chromatography
In normal-phase column chromatography, the selection of an appropriate mobile phase is of utmost importance to achieve the desired separation. The mobile phase is usually a non-aqueous mixture of organic solvents.
Generally, an ideal mobile phase should possess the following properties for its suitability.
- low viscosity
- compatible with the available detection system
- Purity in its state
- low inflammability and toxicity
- high inertness
- sufficient solubility for the solutes being analyzed
The mobile phase consists of dehydrated nonpolar organic solvents, including iso-octane, methylene chloride, methanol, ethanol, 2-propanol, acetonitriles, ethyl acetate, tetrahydrofuran, carbon tetrachloride, and hexane. It may also utilize a binary or tertiary solvent system, combining two or three of these non-polar organic solvents.
Normal phase columns
As previously mentioned, normal phase columns consist of polar stationary phases, and it interacts strongly with polar compounds present in samples. Some of the commercially available normal-phase columns in chromatographic technique are listed below:
- Silica normal phase column: The diameter of this column ranges from 2.1 mm- 4.6 mm.
- Amino normal phase column: Diameter ranges from 2.1 mm- 4.6 mm and Carbon load (2%)
- Cyano normal phase column: Diameter size ranges from 1 mm- 4.6 mm and carbon load (4%)
Applications of Normal phase column chromatography
- This type of technique is mainly used for:
- separation of water-sensitive compounds
- separation of geometric isomers (Cis-Trans isomers) and positional isomers.
- separation of chiral compounds
- This technique is suitable for the analysis of compounds prone to hydrolysis because it employs nonaqueous solvents.
- It has been used to analyze extremely hydrophobic compounds like polyaromatic compounds.
Problem in Normal Phase Chromatography
One of the commonly faced problems in normal-phase chromatography arises due to the insolubility of very hydrophilic compounds in the solvents used for the mobile phase. This problem can be solved using a special type of normal phase column with aqueous mobile phases.






