Table of Contents
ToggleAtomic absorption spectroscopy (AAS) is an absorption spectroscopic method that uses the absorption of light by free atoms in a gaseous state to determine the quantitative composition of chemical components. It is used to determine the concentration of metals present in a sample to be analyzed.
AAS can be used to quantify more than 70 different elements either in solution or solid form and possesses wider applications in clinical analysis, food analysis, the pharmaceutical industry, the mining sector, and so on. Because the atomic absorption method is largely free of interference and the set of electronic energy levels is specific to that element, it is a highly good analytical technique with great sensitivity.
The modern form of AAS was developed by Australian Chemist, Sir Alan Walsh in the 1950s.
Principle of atomic absorption spectroscopy
If a solution containing metal salt (M+X–) is aspirated to the flame, a vapor that contains atoms of metal may be formed. A large number of the gaseous metal atom remains in the ground state, and are capable of absorbing radiant energy of their specific wavelength. If the light of resonance wavelength is passed through the flame containing the atoms which are analyte, the part of the light will be absorbed and the extent of absorption will be directly proportional to the number of ground state atoms present in the flame.
The process by which gaseous metal atoms are produced into the flame can be illustrated as:
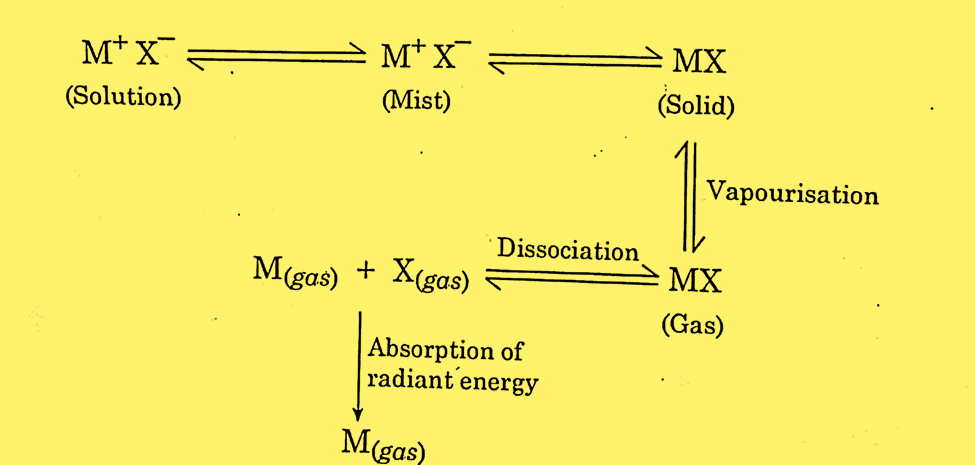
When a metal atom is changed into gas and light is passed from the sources, the ground state of the atom gets excited by absorbing the radiation of a particular wavelength. The absorbance is given by Beer-Lambert’s law; the logarithmic ratio of the intensity of incident light to the intensity of absorbing species.

Atomic absorption spectroscopy instrumentation
The block diagram illustrating the instrumentation of atomic absorption spectroscopy can be shown as:
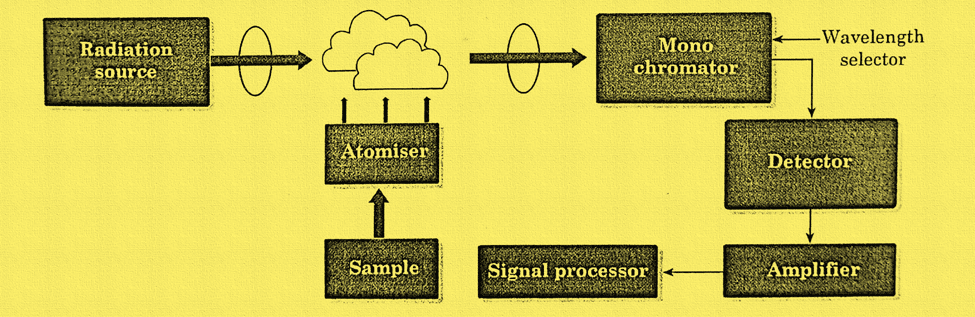
- Atomizers: In order to analyze a sample for its atomic constituents, the element has to be atomized. The atomizers most commonly used nowadays are flames and electrothermal (graphite tube) atomizers. Depending on it, AAS is of two types; Flame-AAS, and electrothermal or graphite furnace-AAS
- Flame-AAS: In a flame atomizer, a solution of the sample is nebulized (sprayed) by a gaseous mixture of an oxidant and a fuel and carried into a flame where atomization occurs.
- Electrothermal or graphite furnace atomizer: Atomization takes place in an open-ended graphite tube in the graphite-furnace AAS process. The material is atomized as the tube’s temperature rises. Radiation enters the tube from one end and excites the analytes. The detector at the other end determines the absorbed fraction.
- Radiation source: A Hollow cathode lamp is the most used type of radiation source. Such a lamp uses low pressure to fill the cylindrical metal cathode with Ar or Ne gas, which contains the desired element as well as an anode. The cathode and anode are subjected to a high voltage, which causes the filled gas to ionize. The cathode material is energized during the glow discharge to release the radiation of the target element as the gas ions are propelled towards the cathode. Other radiation sources used commonly are electrodeless discharge lamps (EDL) and Deuterium lamps (DL).
- Monochromator: In order to distinguish the radiation specifically to each element from other radiation released by the radiation source, the radiation is then passed via a monochromator. It is carried out by using either a prism monochromator or diffracting grating.
- Detector: The monochromator directs the chosen light onto the detector, commonly a photomultiplier tube, which transforms the light signal into an electrical signal proportional to the light intensity.
- Amplifier: The processing of the electrical signal is amplified by the amplifier.
- Read-out: device: On readout devices like meters or digital displays, the output from the detector is appropriately displayed. It has the ability to show absorbance at certain wavelengths as well as the absorption spectrum.
Interferences in atomic absorption spectroscopy
Interference is a phenomenon that causes the intensity of the analyte to change. It decreases the intensity of absorption of light.
Interferences in AAS are categorized into two types:
- Spectral interferences
- Non-spectral interferences
- Matric interference
- Chemical interference
- Ionization interferences
Spectral interference is mainly due to absorption by another closely atomic line or species, and molecular absorbance band close to the spectral line of an element of interest. This issue can be overcome by choosing another line for the analyte, and by measuring and subtracting the background absorption from the total measured absorption to determine the true atomic absorption.
Among non-spectral interferences, matrix interference occurs when a sample is more viscous or has a different surface tension from the standard, which causes variations in sample uptake rate due to changes in nebulization efficiency. By matching the matrix composition of the standard and sample as closely as possible, such interferences can be reduced.
Chemical interference is present when a sample contains a species that, when combined with an analyte, generates a thermally stable molecule that is not entirely broken down by the energy of the flame. Such interference can be deducted by the addition of an excess of another element or compound.
In high flames, ionization interference occurs more frequently. The dissociation process does not stop at the production of ground state atoms. A flame with too much energy can ionize ground state atoms by taking away electrons, which depletes the ground state atoms. By adding an excessive amount of a readily ionized element, a high number of free electrons are produced in the flame, inhibiting the ionization of the analyte and removing ionization interference.
Application of atomic absorption spectroscopy
Some of the major applications of atomic absorption spectroscopy are:
- Analysis of magnesium and calcium in tap water (water analysis).
- Determination of amount of trace elements in contaminated soil (soil analysis).
- Clinical analysis: Estimation of metals in biological fluids such as blood, urine, serum, etc.
- Environmental analysis
- Trace elements analysis in foods, cosmetics, hair, etc.
- Mining: The amount of metal such as gold can be determined in rocks.
- Pharmaceuticals: The amount of catalyst used may be present in trace amounts in final pharmaceuticals. Thus, AAS is used to determine the amount of catalyst present.
Advantages of atomic absorption spectroscopy
- Easy to use
- Cheap
- Rapid
- Greater sensitivity
- Efficient atomization
- Even small quantities of the sample can be analyzed (5-50μL).
- Samples either in solid or slurry or solution form can be analyzed.
Disadvantages of atomic absorption spectroscopy
- Fails to detect non-metals
- Simultaneous analysis of elements is not possible
- Only able to detect 70 elements excluding earth metals
Difference between flame photometry and atomic absorption spectroscopy
Atomic absorption Spectroscopy Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- D.A. Skoog, F.J. Holler, and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.






