Table of Contents
ToggleInductively coupled plasma atomic emission spectroscopy, also known as ICP-AES or ICP-OES is a form of emission spectroscopy that makes use of ICP (Inductively coupled plasma) to excite atoms or ions that produce electromagnetic radiation with a wavelength specific to a given element. The concentration of the element in the sample can be determined by the intensity of this emission.
Plasma Atomic Emission Spectroscopy
Atomic emission spectroscopy measures the optical emission that has been excited from atoms quantitatively to determine the analyte concentration. A highly ionized conducting gas cloud made up of ions, electrons, and neutral particles are referred to as plasma. Over 1% of the total atoms in a gas are usually ionized in plasma. The last 35 years have seen significant advancements in the use of plasma as an atomization and excitation source for emission spectroscopy, broadening the application of atomic emission spectroscopy (AES). Plasma source improves the accuracy, sensitivity, and precision for a large number of elements over that found with other sources.
In plasma emission spectroscopy, argon is typically ionized under the influence of an electric field via radiofrequency or direct current. Direct current plasma (DCP) and inductively coupled plasma (ICP) are the two forms of discharge that generate plasma. Here, the temperature ranges from 7000 to 15000K. More excited emitted atoms are produced by the plasma source, particularly in the UV region, than by the relatively low temperature utilized in flame emission spectroscopy.
Furthermore, the plasma source is able to reproduce atomization conditions with a far greater degree of precision than obtained by classical arc and spark spectroscopy. As a result, many different elements’ spectra are generated, making the plasma source appropriate for multiple elemental analyses simultaneously. This characteristic is essential for multi-element determinations across a broad concentration range.
Types of plasma atomic emission spectroscopy
1. Direct current plasma(DCP)
High voltage discharge takes place between two graphite electrodes in this process. The most recent design increases the stability of the discharge by using a third electrode (tungsten-cathode) positioned in an inverted Y-shape. The sample is nebulized using argon as the carrier gas at a slow rate of 1mL/min. The high-voltage discharge can maintain a current of about 20 A indefinitely in the ionized argon. In general, the DCP’s detection limits are lower than those of the ICP. The graphite electrodes in the DCP need to be replaced after a few hours of operation, despite the fact that it is relatively less expensive and uses less argon than the ICP.
The process involves the Formation of the liquid droplets, desolvation, atomization, excitation, and emission of radiation at a specific wavelength followed by measurement of the intensity of emitted radiation – using a spectrometer, which contains a monochromator, detector, amplifier, and read-out device.
2. Inductively coupled plasma (ICP)
Inductively coupled means that the plasma is generated due to differences in the magnetic field and currents.
Three concentric silica quartz tubes make up an inductively coupled plasma (ICP) source, and the middle tube is where the argon stream that carries the sample in the form of an aerosol passes through. Two or three turns of a metal induction tube, through which a radiofrequency current with a frequency of around 27 MHz passes, serve as the excitation source. The plasma is maintained by the second flow of argon at a rate of 10-15 Lmin-1. The radiofrequency power excites this gas stream in this manner.
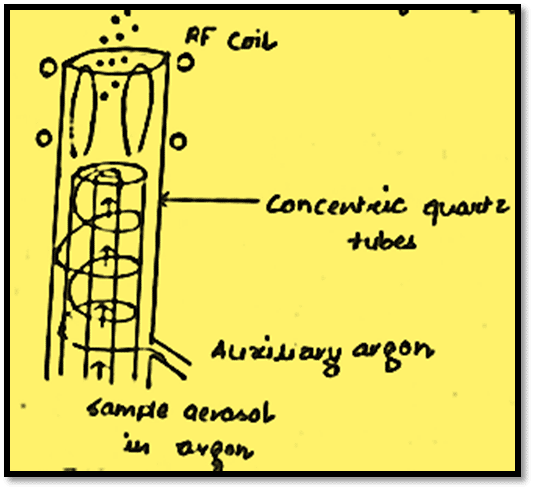
The helical structure of the plasma gas flow provides stability and aids in the thermal isolation of the outermost quartz tube. The plasma is initiated by a spark from a tesla coil probe and is thereafter self-sustaining.
Principle of Inductively coupled plasma atomic emission spectroscopy
Inductively coupled plasma atomic emission spectroscopy (ICP-OES or ICP-AES) is an analytical spectroscopic technique that relies on optical emission for analysis and gives information about how much of certain elements are in a sample. It is widely used to analyze liquid samples as well as substances that are easily dissolved or digested into liquid form.
Since atoms and ions can absorb energy, the ICP-OES principle makes use of this property to shift electrons from their ground state to an excited state. It depends on those excited atoms emitting light at particular wavelengths as they undergo a transition to low energy levels. In layman’s terms, an electron produces light of a specific wavelength as it undergoes transitions from a higher energy level to a lower energy level, typically the ground state. The wavelength of the light emitted varies depending on the type of atom or ion (i.e., whatever element it is) and the energy levels the electron is moving between.
The quantity of atoms or ions making that are responsible for the transition determines how much light is released at each wavelength. The relationship between light intensity and element concentration is described by the Beer-Lambert law.
Inductively coupled plasma atomic emission spectroscopy instrumentation
In flame photometry, atomization is influenced by flame, in AAS by electrothermal atomizers or flame, and in ICP-AES by an ICP-torch with this difference. The rest of the techniques are the same as those employed in flame photometry or AAS.
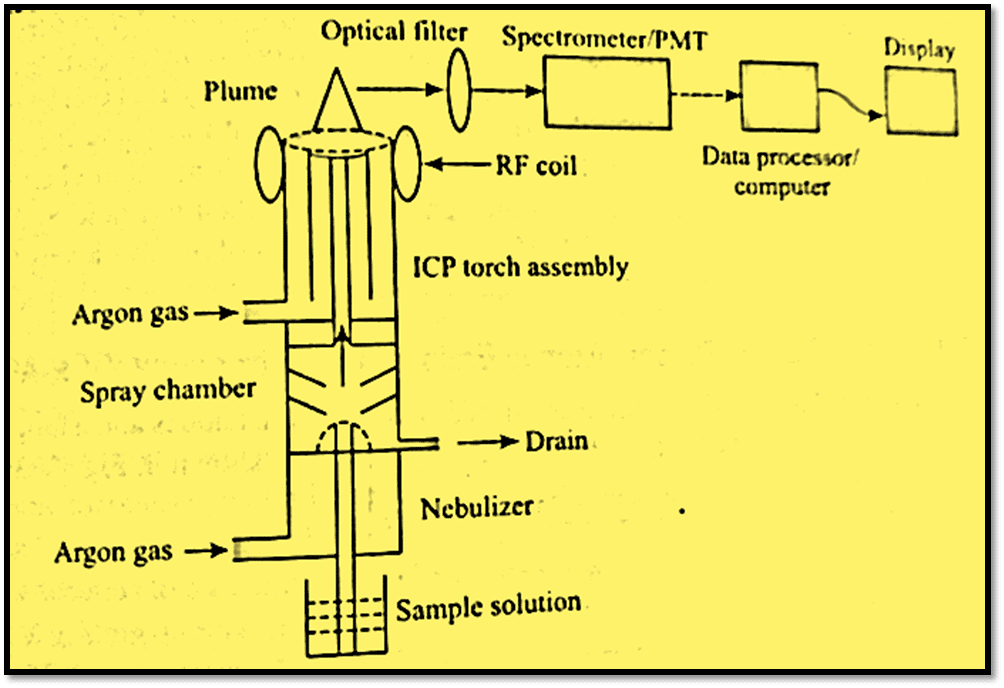
The major components involved in Inductively coupled plasma atomic emission spectroscopy (ICP-AES) are:
- Nebulizer: It is a device that turns liquid into tiny particles called aerosol that can be carried to the plasma. The sample’s droplets are broken up using ultrasonic forces.
- Pumps: Peristaltic pumps, which do not come into touch with the liquid, are used to pump liquid into the nebulizer.
- Sprayer: The spraying chamber is used to transport liquid droplets into the plasma. It is located between the nebulizer and torch and removes large droplets.
- Sample injector: It contains the sample and pumps it to the nebulizer. As, Bi, Ge, and Sn are produced using a hydride generator.
- Radiofrequency generator: This is a device that generates power for plasma discharge. The RF generator’s frequency is between 27 and 56 MHz, and its power ranges from 600 to 1000 W.
- Detector: usually a photomultiplier tube is used to measure the intensity of the emitted radiation. It is capable of amplifying an electrical signal by a combination of dynodes.
- Read-out: The data is displayed on readout devices.
Interferences in ICP-OES
Some of the commonly observed interferences in Inductively coupled plasma atomic emission spectroscopy (ICP-OES/ICP-AES) are:
- Spectral Interference: It results from the crossing over of a spectral line from a different element or from background emission from ongoing or recombination processes. Either eliminating background emissions derived from observations near the analyte wavelength peak or inter-elemental correction can be used to eliminate this interference.
- Chemical Interferences: Such interferences are highly dependent on matrix type and specific analyte elements.
- Physical Interferences: The factors like surface tension, viscosity, organic solvents, etc, cause interferences, and can be removed by use of the internal standard.
Advantages of Inductively coupled plasma atomic emission spectroscopy
Some of the major advantages of inductively coupled plasma atomic emission spectroscopy are:
- Good sensitivity even at low concentration
- Highly specific
- Simultaneous multi-elemental analysis
- Identification of metalloids.
- Samples in either liquid or solid state can be analyzed.
Applications of Inductively coupled plasma atomic emission spectroscopy
It is an extremely complicated approach for the examination of several heavy metals, metalloids, and even non-metals (at ppm to ppb level) in a wide range of materials that are derived from various sources. This technique possesses greater applications in biological, chemical, and environmental sciences. Thus, some of the major applications of inductively coupled plasma atomic emission spectroscopy are
- For the determination of arsenic in food, and trace elements bound to proteins.
- ICP-AES techniques can be employed for the analysis of agricultural products and foods.
- For the clinical analysis of metals in biological fluids (blood, urine) eg. Al in blood, Cu in brain tissue, and Se in the liver.
- Environmental analysis of trace metals and other elements in waters, soils, plants, composts, and sludges.
- In Forensic science; gunshot power residue analysis, toxicological examination, and so on.
- Trace metal analysis in raw materials.
- Inductively coupled plasma atomic emission spectroscopy is also used for motor oil analysis.
Inductively Coupled Plasma Atomic Emission Spectroscopy Video
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- D.A. Skoog, F.J. Holler, and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition.






