Table of Contents
ToggleThere are numerous properties of water including chemical and physical properties which are very essential for life on earth.
What is water?
Water is an important monoxide of hydrogen. It contains hydrogen and oxygen atoms in the ratio of 2:1; hence, its formula is H2O. It is an essential constituent of all living cells. Lavoisier proved that water is composed of hydrogen and oxygen. Nearly 75% of the earth’s surface is covered by water. Water is present in three physical states on earth, a solid; in the form of ice, liquid; in the form of water in the lakes, and vapor; in the form of water vapor.
Physical Properties of water
- Pure water is transparent, colorless, tasteless, and odorless. Water in a thick layer appears greenish blue.
- Pure water is a bad conductor of heat and electricity. However, the addition of a small quantity of acid, alkali, or salt makes it conduct.
- It has a maximum density of 4oC (1gm/cc).
- Water has a higher density than ice. Due to this, ice floats on water. This is useful for the survival of aquatic life at the bottom of lakes and rivers.
- Water has a high dielectric constant (82). It dissolves most of the ionic compounds, hence the name “universal solvent.
- Due to the high heat capacity of water, it can absorb heat either from physiological reactions or automobile engines.
- It has a high latent heat of fusion (334 J/gm) and latent heat of evaporation (2257 J/gm).
- Due to the presence of intermolecular hydrogen bonding among water molecules, water has abnormally high m.p. and b.p. As compared to the hydrates of other members of group VI A of the periodic table.
Structure of Water
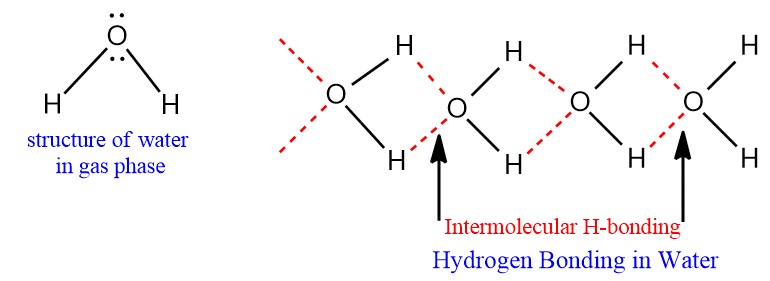
Water molecule is a covalent compounds. It contains two hydrogen atoms covalently bonded with central oxygen atoms. In a water molecule, its central atom undergoes sp3 hybridization. It is assumed to be the tetrahedral shape of the molecule in which two H atoms should occupy two concerns of the tetrahedron and the remaining two corners should occupy two lone pair electrons of the oxygen atom. But according to the valance shell electron pair repulsion model, lone pair-lone pair repulsion is greater than that between lone pair-bond pair and bond pair-bond pair repulsion.
Consequently, the H2O molecule has a bent structure with an HOH bond angle decreasing from 109.5o (normal tetrahedral bond angle) to 104.5o. Hence, when atomic nuclei are considered, its v-shaped or bent structure;
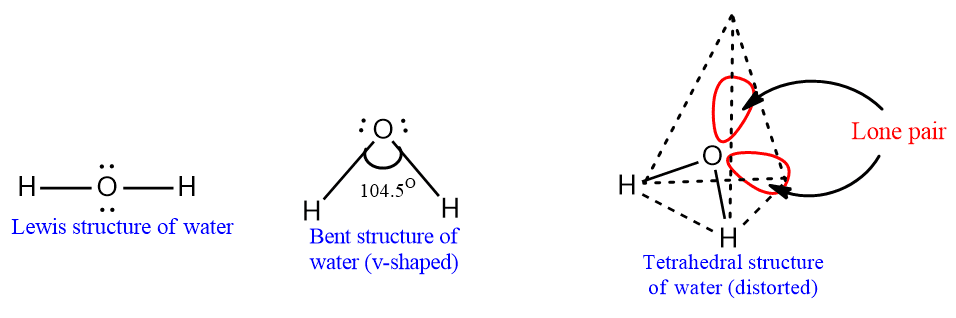
It is observed that oxygen has large electronegativity than hydrogen. Due to the strong electronegative character of an oxygen atom, the water molecule is highly polarized, i.e. partial positive charge on the H atom and a partial negative charge on the O atom. As a result, intermolecular hydrogen bonding between the oxygen atom of one water molecule and the hydrogen atom of another water molecule takes place.
From X-ray studies, it is known that water molecules in ice arranged to form loose open cage-like structures with vacant spaces perhaps due to hydrogen bonding.
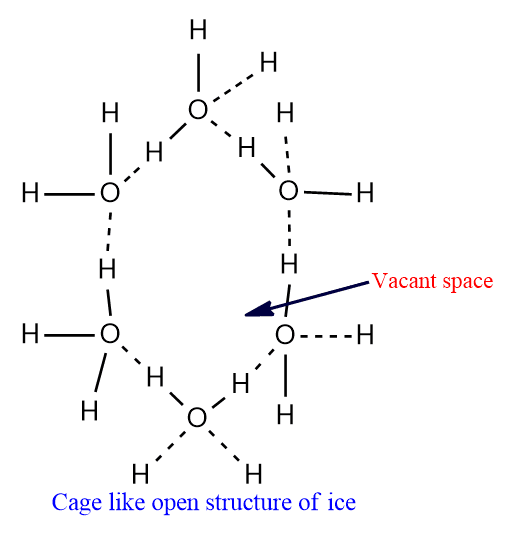
When ice is melted, some of the hydrogen bonds are broken and the open cage-like structure is partially destroyed causing the molecules to come closer to each other.
Chemical properties of water
- With litmus paper: It is neutral to litmus paper.
- Self-Ionization of water: It is a weak electrolyte. It partly ionizes into H+ and OH– ions. The H+ ion gets hydrated as the H3O+ ion (hydronium ion).
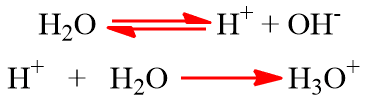
For simplicity, H+ ion is written instead of H3O+. Pure water is neutral due to an equal concentration of H+ ion and OH– ions i.e. [ H+] = [OH–] = 10-7 mol/L.
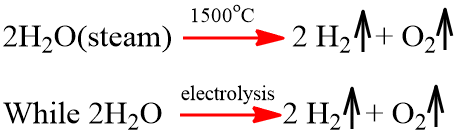
- Decomposition: H2O is quite stable towards heat but decomposes about 1% at 1500oC.
- Amphoteric Nature: Water behaves as an acid as well as a base in reaction.

- Oxidizing and Reducing agent: H2O acts as a weak oxidant as well as a weak reductant.
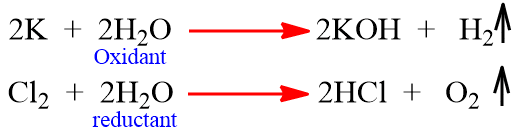
- Hydration reaction: Water combines with many salts to form hydrated salts.

Anhydrous copper sulphate is white which changes to bluish-green with water. Therefore it is used as a test for water or moisture.
Related video