Table of Contents
ToggleHow to read IR spectra? Everybody has to face some difficulty while analyzing due to a lack of basic ways or tricks. If you don’t know about the basics of IR spectroscopy, you can read.
Before discussing the basic idea to analyze the IR spectra, we must know about the regions present in the spectra. Do you know, how many regions are there in IR spectra? Actually, there are two regions: fingerprint region and diagnostic region.
The fingerprint region ranges from 400 to 1500 cm-1 and the diagnostic region ranges from 1500 to 4000 cm-1. The fingerprint region is unique for each and every compounds just like the fingerprint of humans. But diagnostic regions have some characteristic regions. By observing these we can identify which types of bond or functional group is present. Before going to these regions, let’s study the absorption frequency of each bond or functional group.
Note: Two or more compounds may have the same diagnostic region but different fingerprint regions. Therefore, two identical compounds have exactly overlapping spectrum.
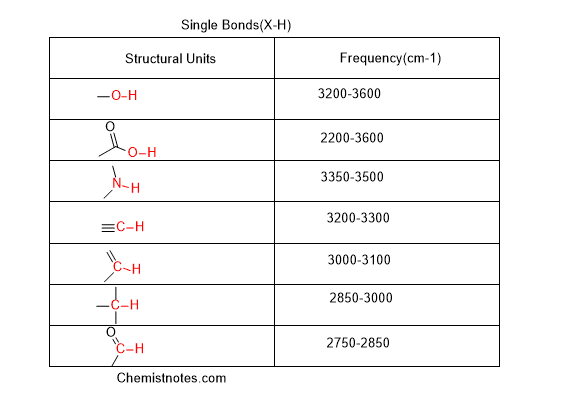
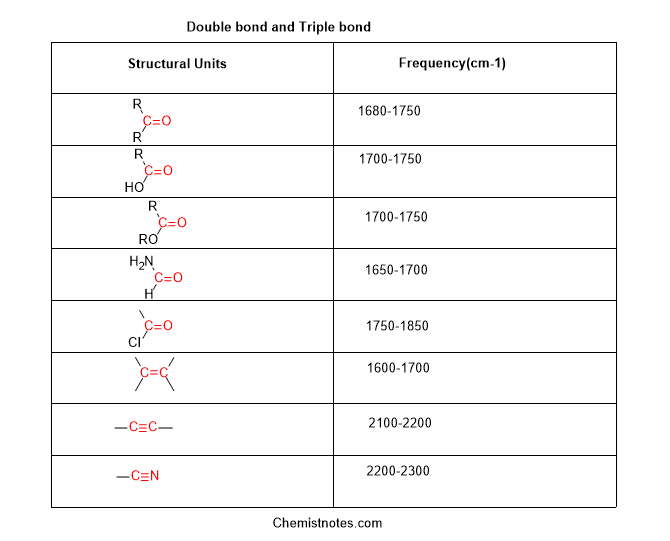
Now, remember these values of absorption frequency. But we will try to identify the peak by observing the shape of the peak and the location of that peak in the spectra. Are you ready for the trick? Ok, let’s move to some easy tricks to identify the spectra.
Step 1: Look at the diagnostic region first(above 1500 cm-1)
As we know, the fingerprint region is very complex, it is difficult to read or identify. Therefore, we will try to identify the spectra by observing the diagnostic regions.
Step 2: Look at double bond region(1600-1800 cm-1)
In this region, the signal of double bonds such as carbonyl group(C=O) and a carbon-carbon double bond(C=C ), and a benzene ring is observed.
Peak shape= sharp sword-like peak.
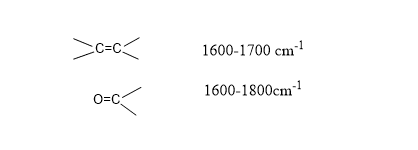
If a sword-like peak is present in the range of 1600-1800 cm-1 then the compound must have either C=O group or C=C group.
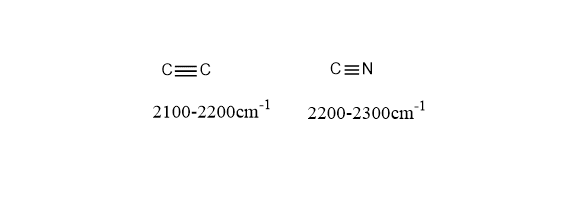
Step 3: Look at triple bond region(2100-2300 cm-1)
If you observe any peak in the range of 2100-2300 cm-1 then the compound has triple bonds such as C≡C(2100-2200 cm-1) and C≡N (2200-2300 cm-1).
Step 4: Draw a line at 3000cm-1 and observe signal above and below it
The borderline 3000 cm-1 contains small peak above or below it.
- If single small peak is present around 2800-3000 cm-1, we can say thats the peak due to sp3 C-H bond( in alkane)
- If small signal peak is present around 3000-3100 cm-1, then we can say thats the peak due to Sp2 C-H bond( in vinylic C-H bond).
- If small peak around 3300 cm-1 is present then thats the peak due to Sp C-H bond( in alkyne).
Step 5: Is there any tongue like peak? Observe it.
- If small tongue like peak is present near 3200-3400 cm-1 then its due to O-H bond.
- If broad tongue like peak is present near 2200-2600 cm-1 then its due to COOH group.
Note: The peak due to the COOH group is broad than the OH bond because of hydrogen bonding. To distinguish these, look at the double bond region where a sharp peak of C=O must present in a carboxylic acid but not in alcohol.
Step 6: Observe any fang like peak above 3300 cm-1.
- If two fang like peak is present around 3500 cm-1, the compound may be primary amine or primary amide. To distinguish these, look at double bond region where a sharp peak of C=O bond is present in amide.
- If a single peak around 3500cm-1 is present then the compound may be secondary amine or secondary amide.
Step 7: Confirm the structure of compound.
The structure of the unknown compound is then confirmed by comparing the spectra of the standard compound.
Remember 5 main regions/Peaks in the spectra
- Tongue like peak(3200-3400 cm-1)
- Sword like peak (1600-1850 cm-1)
- Borderline around 3000 cm-1
- Triple bond region (2100-2300 cm-1)
- Fang like structure around 3500 cm-1
Let’s analyze some spectra of unknown compounds and try to find out important information from the spectra.
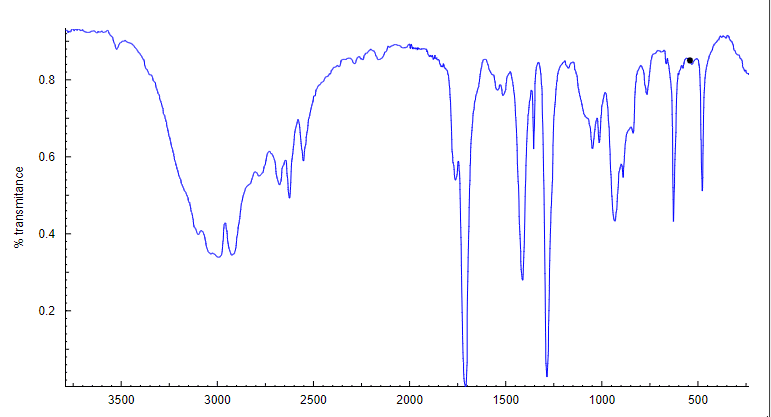
Steps:
- First of all, observe the spectra above 1500 cm-1.
- Observe double bond regions. In above spectrum, we can see one sharp sword like peak around 1700 cm-1. Its means compound has carbonyl group.
- Observe triple bond region. In the above spectrum, we can see there isnot any peaks. It means compound has not triple bond.
- Then observe around border line 3000 cm-1. In above spectrum, we can see a small peak is present around 2900 cm-1. It means compound have C-H bond(sp3 carbon-hydrogen bond),
- After then, observe any fang or tongue like broad peak. In above spectrum, we can see a broad tongue like peak is present from 2200-3500 cm-1. It means the compound contains carboxylic group(-COOH group).
Do you understand? Alright, this is the basic way to read the IR spectrum of any given compounds. Comment down if you have any problems regarding this topic. Thank you.
You can also get some idea or tricks about the reading of NMR spectrum(click here).