Table of Contents
ToggleCommercial cells are the source of energy. They are electrochemical devices that are used as a source of energy, and they provide a relatively constant voltage over time. Batteries are formed when two or more commercial cells are connected in series. These batteries are used to produce portable electrical energy. Thus, a battery is an electrochemical cell arrangement used as a source of electrical power.
The following criteria should be included in a useful battery:
- It should be light and compact so that it can be easily transported.
- It should have long life both when it is used and when it is not used.
- The voltage or potential of the battery should not vary appreciably during its use.
Types of Commercial Cell
There are mainly three types of commercial cells.
- Primary cell
- Secondary cell
- Fuel cell
Primary Cell or Battery
It involves only galvanic or voltaic cells. A primary cell is an electrochemical device in which a chemical reaction occurs spontaneously and an electric current is produced. In these cells, the electrode reaction can not be reversed by external electric energy. These cells only undergo one reaction, and after use, they become dead. Therefore, they are not chargeable. Hence, primary cells are also called use and throw cells. example: dry cell, bottom cell, mercury cells, etc.
Dry cell (the Lechlanche cell)
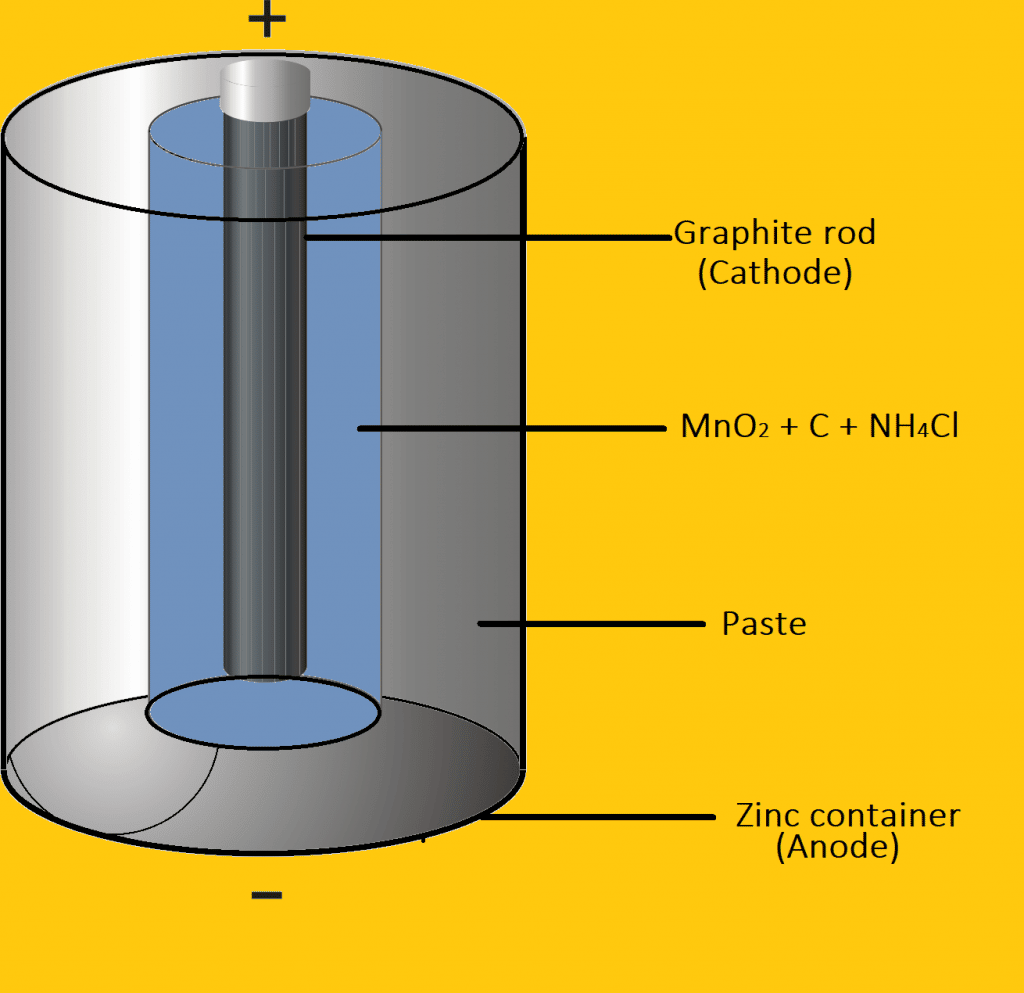
The most familiar commercial cells are dry cells. These are used in torches, toys, calculators, tape recorders, and many other devices. The dry cell consists of a Zn cylinder which is filled with a paste of NH4Cl and ZnCl2. The zinc cylinder act as an anode and the carbon rod is surrounded by a black paste of MnO2 and carbon powder acts as a cathode. When the cell is working, Zinc loses e- and produced Zn+2 ion which is dissolved in the electrolyte. The electrons pass around the external circuit and are taken up at the cathode. This cause the discharge of NH4+ ion from the electrolyte.
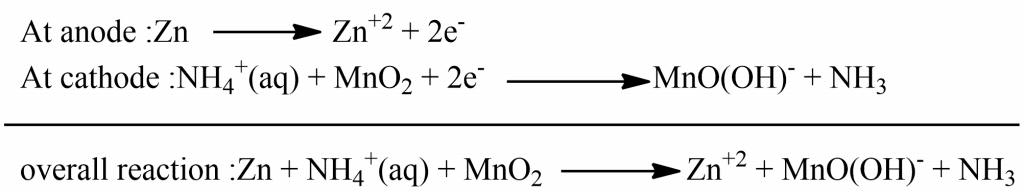
Thus formed ammonia is not liberated as gas but it combines with the Zn+2 ion and produces a complex ion as [Zn(NH3)2]+2. This dry cell does not have an indefinite life because NH4Cl is acidic in nature and corrodes the Zn containers even when it is not in use.
Secondary Cell
It involves both galvanic and electrolytic cells. They are galvanic cells (electrochemical cells) during discharge but are electrolytic cells during recharging. In the secondary cell, the reaction can be reserved by external electrical energy. Therefore, these cells can be recharged by passing an electric current and used again and again. On recharging, they store electrical energy in the form of chemical energy. Therefore, they are called storage cells. e.g, a lead storage cell, Ni-Cd cell.
Lead storage cell
Such a type of cell consists of a number of voltaic cells connected in a series. Generally, 3-6 such cells are combined to get a 6-12V battery. In such a cell, the Pb rod acts as an anode and the grid of Pb packed with PbO2 acts as a cathode. An aqueous solution of 30-40% H2SO4 having a specific gravity of 2.15 acts as an electrolyte. The following are the electrode reaction during discharge:
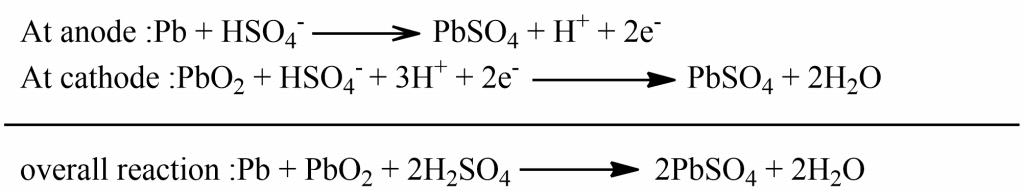
Just the opposite reaction occurs during recharging.
Difference Between Primary Cell and Secondary Cell
| Primary cell | Secondary cell |
| It involves only galvanic or voltaic cells. | It involves both galvanic and electrolytic cells. |
| In the primary cell, a chemical reaction occurs spontaneously and an electric current is produced. | In the secondary cell, the reaction can be reserved by external electrical energy. |
| They are not chargeable. | These cells can be recharged by passing an electric current. |
| It is also called dry cells. | These are composed of molten salt and wet cells. |
| It has a high internal resistance. | It has a low internal resistance. |
FAQs
What is a primary cell?
A primary cell is an electrochemical device in which a chemical reaction occurs spontaneously and an electric current is produced.
What is a secondary cell?
The secondary cell is where the reaction can be reserved by external electrical energy. Therefore, these cells can be recharged by passing an electric current and used again and again.






