Table of Contents
ToggleElectronegativity is the ability of a neutral atom in a stable molecule to attract electrons. In 1931, Pauling defined the electronegativity of atoms as the tendency of the atom to attract electrons to itself when combined in a compound. Generally, small atoms attract electrons more strongly than large ones, and hence small atoms have more electronegativity. Atoms with nearly filled shells of electrons tend to have higher electronegativities than those with poorly occupied ones.
Electronegativity values are very difficult to measure. It is not an absolute quantity; it is only a relative property of an atom in a molecule in the environment and under the influence of surrounding atoms. It is not a precise quantity, but rather a relative value. Pauling and others have attempted to relate the electronegativity difference between two atoms to the amount of ionic character in the bond between them. Some of the more important approaches to obtaining electronegativity values are;
- Pauling’s approach (scale) of electronegativity
- Mulliken’s scale of electronegativity
- Allerd and Rochow scale of electronegativity
Pauling’s approach (scale) of electronegativity
On the basis of electronegativity, Pauling explains the fact that the covalent bond between two different atoms (A-B) is stronger than would be expected by taking the mean of the bond energies of A-A and B-B bonds. The Pauling scale of electronegativity is based on the bond energy of the molecule.
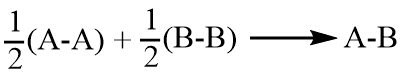
According to Pauling, for a covalent molecule (say A-B), its bond energy is equal to the geometric mean of bond energy of A-A and B-B molecule.
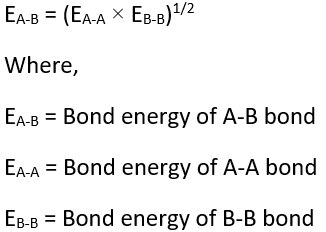
It has been found that the experimental (actual) bond energy is always greater than its theoretical bond energy. This excess bond energy is called ionic covalent resonance energy. This extra bond energy, i.e., the difference in bond energy, is denoted as delta (Δ) and given by the following empirical formula.
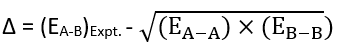
The resonance energy of the molecule depends on the polarity of the bond, which in turn depends on the electronegativity difference between two atoms, A and B. Let χA and χB denote the electronegativities of A and B respectively.
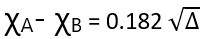
Here Δ is expressed in KCal mol-1. However, if Δ is expressed in eV per molecule then the above expression becomes.
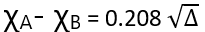
The extrastability of the A-B bond originates from the polarity developed in it due to the difference in electronegativities of A and B.
| H 2.1 | ||||||
| Li 1.0 | Be 1.5 | B 2.0 | C 2.5 | N 3.0 | O 3.5 | F 4.0 |
| Na 0.9 | ||||||
| K 0.8 | ||||||
| Rb 0.8 | ||||||
| Cs 0.7 |
If two atoms have similar electronegativities, that is a similar tendency to attract electrons, the bond between them will be predominantly covalent.
Mulliken’s scale of electronegativity
In 1934, Mullikens suggested an alternatiive approach to electronegativity based on electron affinity and the ionization energy of an atom. According to Mullikens electronegativity is the airthmetic mean of ionization energy and electron affinity of an atom, both expressed in electron volts (eV).
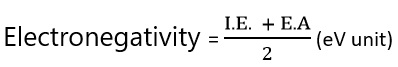
If I.E and E.A are expressed in KJ /mole, the above relationship may be written as,
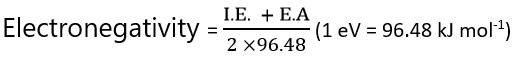
Mulliken values of electronegativity are about 2.8 time greater than as pauling’s value. Hence, to adjust Mulliken’s value with Pauling’s values, the expression will be,
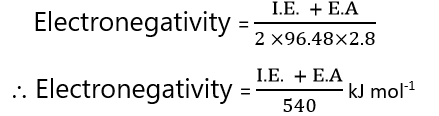
Allered and Rochow scale of electronegativity
Allered and Rochow scale of electronegativity depends on the effective nuclear charge. It is widely adopted empirical approach to electronegativity. They defined electronegativity as the attractive force between a nucleus and an electron. This force is electrostatic and given by,
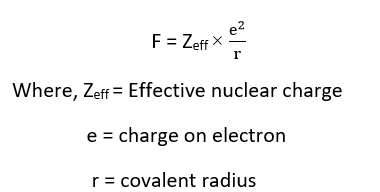
This force of attraction, F, may be converted in to electronegativity values of using empirical formula.
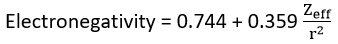
The electronegativity of an element mainly depends upon hybridization of valance state an d atomic charges.
Relation between Hybridization and Electronegativity
The electronegativity of an atom vary slightly with hybridization, with those orbitals having greater s-character being more electronegative.
sp > sp2 > sp3
This is because the percentage of s-character in these hybrid orbital are 50%, 33.33%, and 25% respectively.
Examples:
H-C ≡ C-H > H2C = CH2 > CH3 – CH3
Relation between Atomic charge(oxidation state) and Electronegativity
The electronegativity increases with the increase in positive charge of an atom, while it decreases with increases in negative charge. It is because the tendency to attract electrons increases with the increase in positive charge and vice versa.
Examples:







