Table of Contents
ToggleThe word carbohydrates are derived historically from the idea of “hydrates of carbon,” the general formula Cn(H20)n; for example, glucose has the formula C6H1206. Sucrose (common table sugar) is
consistent with another general formula, Cn(H20)m, because it has the formula C12(H20)11. Glucose, cellulose, starch, and glycogen all belong to the class of organic compounds known as carbohydrates. Carbohydrates are the ultimate source of most of our food: we eat starch-containing grain or feed it to animals to be converted into meat and fat which we then eat. Carbohydrates are polyhydroxy aldehydes, polyhydroxy ketones or compounds that can be hydrolyzed to them.
Test for carbohydrates
Carbohydrates do not have uniformly recognizable properties. These compounds are usually water-soluble solids that melt with decomposition; these characteristics correctly point toward the presence of a large number of highly polar functional groups in these molecules.
Molisch’s test
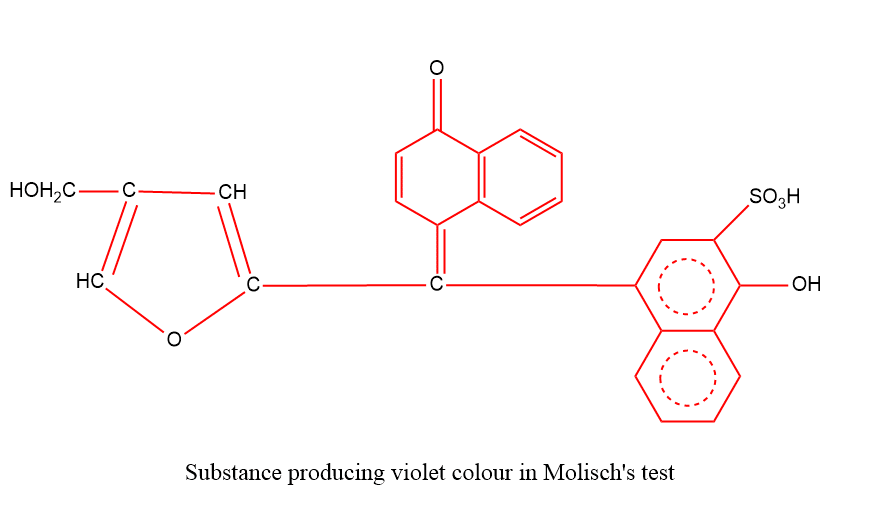
To 2 ml dilute aqueous solution of a substance in a test tube add 2-3 drops of Molisch’s reagent (10% α-naphthol in alcohol) and then pour 1 ml conc. H2SO4 from the side of the tube cautiously without disturbing the contents. The appearance of a violet ring at the junction of two liquids which on shaking produces deep violet solutions indicates that the compound is a carbohydrate.
The first step in the appearance of color is the formation of furfural or its derivatives by splitting of the carbohydrate by concentration sulphuric acid, followed by dehydration. Furfural or its derivative then condenses with α-naphthol forming a colored product of the following probable structure:
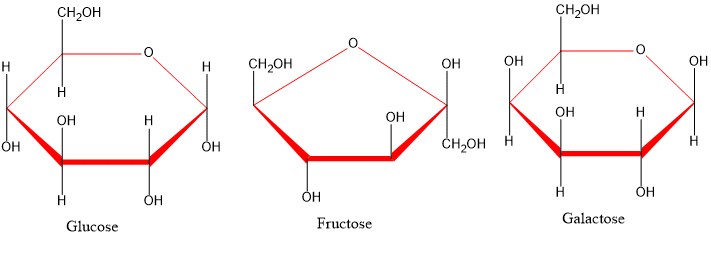
Glucose and fructose (monosaccharides), sucrose, lactose, and maltose (disaccharides) respond to this test.
Benedict’s test
To 2ml of Benedict’s reagent, add 0.2ml of a 2% solution in water of the unknown. Boil the mixture and then allow it to cool. A red ppt forms with reducing sugar, while the solution remains clear with a non-reducing sugar.
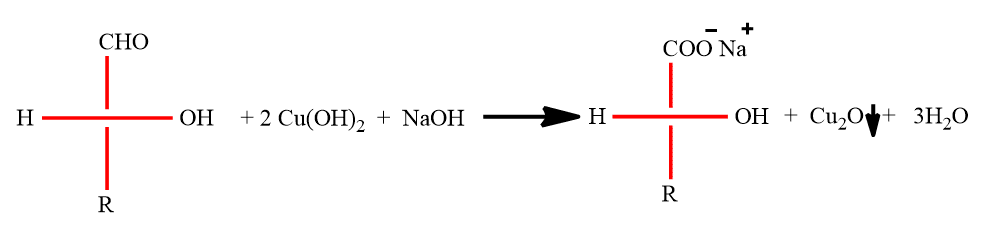
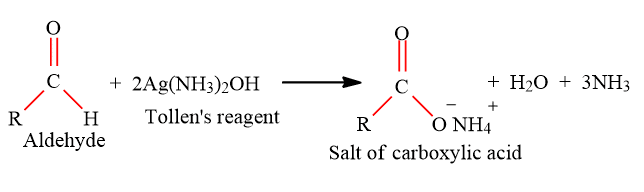
Tollens’ test
Some carbohydrates also respond to Fehling’s test and tollens’ test. Glycosides after hydrolysis with dilute HCl also respond to these tests.
The Tollens test can be used to test for the presence of the aldehyde group. The aldehyde functional group is oxidized to the salt of the carboxylic acid, with the silver ion being reduced to elemental silver, which is deposited as a coating inside the reaction flask.
- In addition glycosides on treatment with cold concentrate H2SO4 produce an intense red color.
Confirmatory test for Carbohydrates
Charring of carbohydrates
If the above test is positive then perform the following test.
In a small test tube heat 0.25 g of the carbohydrate with 1 ml of conc. sulfuric acid. Immediate charring takes place, which indicates a carbohydrate.
Preparation of Osazone
The synthesis of Osazones from sugars and phenylhydrazine, can be utilized to discriminate between the various sugars. According to the types of sugar present, the time required to develop the solid Osazone is different.
To 5 ml of an aqueous solution of a substance in a boiling, tun=be add 10 drops of glacial acetic acid and 5 drops of phenylhydrazine. Shake the contents and keep the boiling tube in boiling water for 5 minutes to 90 minutes(depending on carbohydrate). While shaking the tube at short intervals, wash till yellow or orange-colored solid osazones separates out. Filter wash with water, crystallize from hot water or 60% alcohol. Dry and determine the melting point.
- Osazone formed in four minutes of D-glucose, formed in 2 minutes of fructose, formed in 1 hour of maltose, and formed in 20 minutes of D-galactose.
- The osazones of maltose and lactose are obtained only by cooling the solution after fifteen minutes of boiling.
- Osazones of maltose in white in color.
Glucose
Take 2 ml of the aqueous solution of sugar in a test tube add 50mg of lead acetate and heat to boiling. Add 5 ml of dil. ammonium hydroxide and boil the mixture again for 1 min-rose pink color appears.
Fructose
Take 2 ml of the aqueous solution of sugar in a test tube and add an equal amount of conc. hydrochloric acid and 10 mg of resorcinol warm the tube in a beaker of boiling water for 2 min a deep color appears followed by a precipitate.
Galactose
Place 0.5g of the substance in a dry test tube and add 1.5ml of 50% nitric acid. Allow the tube to stand in a beaker of boiling water until red fumes evolve. Then keep the tube for 15 min in another beaker containing water at 70oC a white ppt results.
Sucrose
Sucrose does not reduce Fehling’s solution. On treatment with conc. sulphuric acid gets charred even in cold. Sucrose also responds to color tests.
Dissolve 0.5 g of the substance in 2ml of distilled water. To this add 0.1g of resorcinol and 1.5 ml conc. hydrochloric acid. Boil the mixture for 2 min a deep wine red color appears.