Table of Contents
ToggleStereoselective reactions are reactions that produce predominantly one stereoisomer (or one pair of enantiomers) out of several possible diastereomers. Which product we obtain depends on which stereoisomers we start with. However, a stereospecific reaction is one in which stereochemically different molecules react differently. “Stereochemically different” molecules are meant to be stereoisomers, i.e., enantiomers or diastereomers, and “react differently,” meaning that they show any difference in chemical behavior.
Some reaction mechanisms
The terms syn-addition and anti-addition are used to characterize the different types of stereochemistry that can occur in addition reactions. These terms are not the names of specific mechanisms, but simply indicate the stereochemical facts: that the added groups become attached to the same face (syn) or to opposite faces (anti) of the double bond.
Mechanism of addition of halogen to an alkene
The addition of halogens to alkenes is believed to proceed in two steps. In step 1st a halogen is transferred, without a pair of electrons, from a halogen molecule to the carbon-carbon double bond; there is formed a halide ion and an organic cation. In step 2nd this cation reacts with a halide ion to yield the addition product.
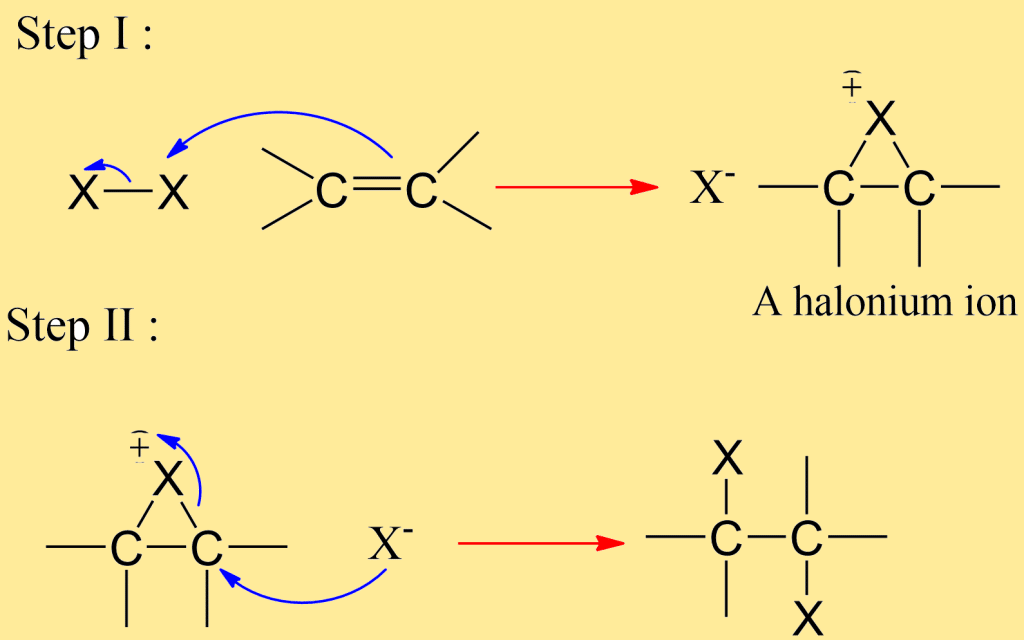
Now, our main concern, in this case, is the nature of the intermediate cation. It is a halonium ion, which is a cyclic ion with halogen linked to both carbon and a positive charge. Halogen adds with complete stereoselectivity and in the anti sense.
Addition of bromine to alkenes
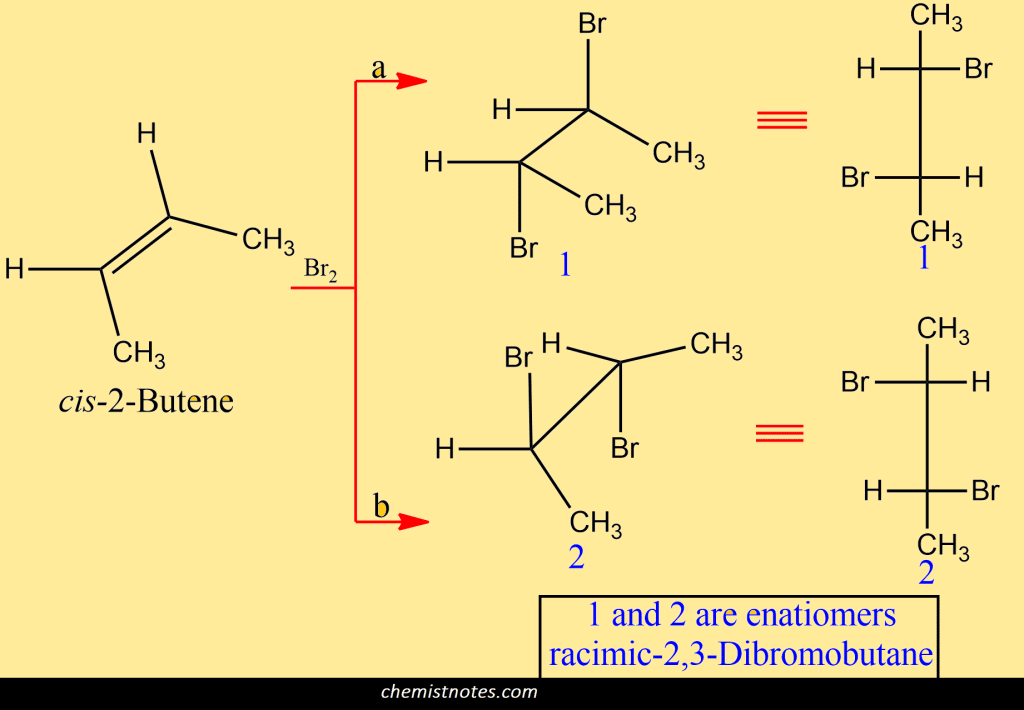
The addition of bromine to alkenes is both stereoselective and stereospecific. If the product forms are stereospecific steroisomeric alkenes react differently and give different products. The addition of bromine to the 2-butenes involves anti-addition. Let us see that this is so. If we start with cis-2-butene, we can attach the bromines to the opposite faces of the double bond in two different ways. From path a gives enantiomer 1 and from path b give enantiomers 2. From this type of reaction mechanism, we obtain the racemic modification.
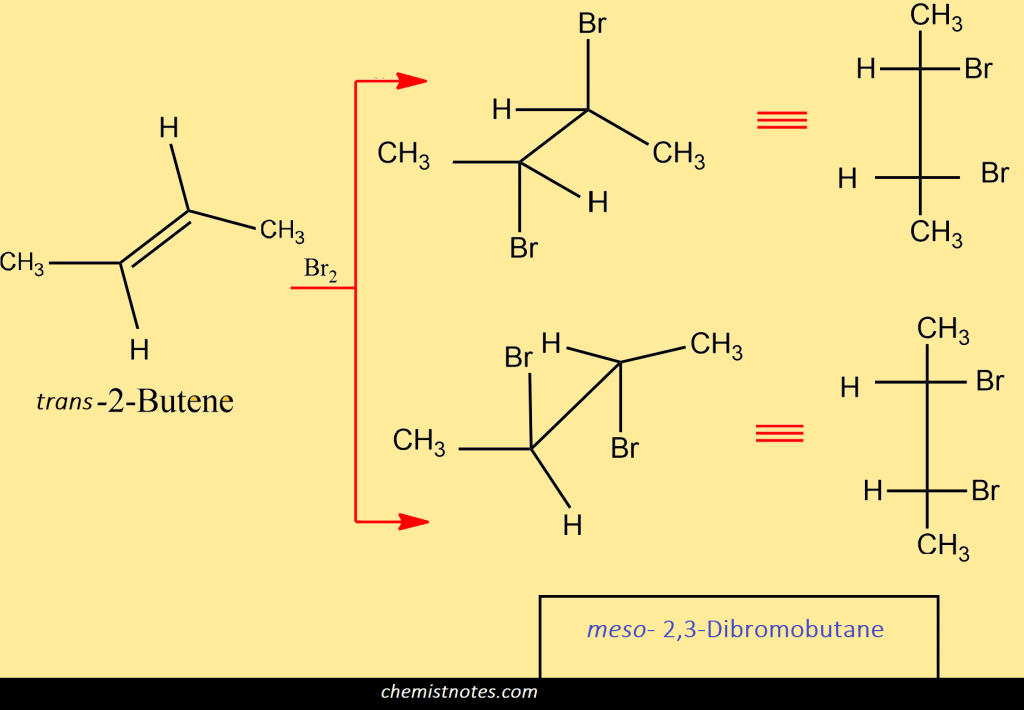
Again, let’s start with trans 2-butene; here also react bromine to opposite faces of the double bond in two ways. From both ways, we found the same product which is the meso dibromide.
Anti-addition is the general rule for the reaction of bromine or chlorine with a simple alkene.
Stereochemistry of the E2 reaction
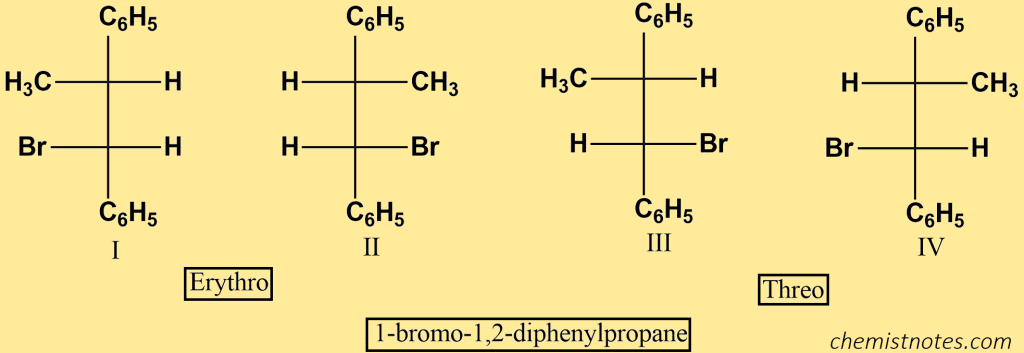
Using dehydrohalogenation under E2 circumstances, let’s examine the stereochemistry of elimination. Consider dehydrohalogenation of the alkyl halide 1-bromo-1,2-diphenylpropane.
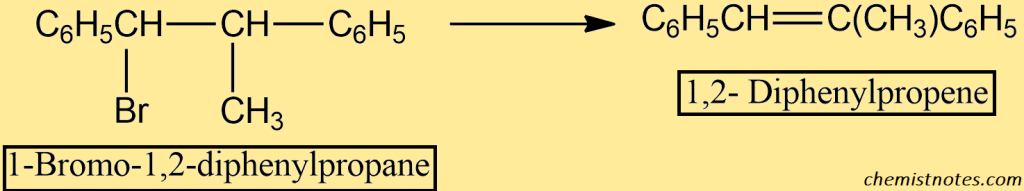
This compound contains two chiral centers, and we can easily show that it can exist as two pairs of enantiomers. I and II are called erythro; and III and Iv, are called threo. Each pair is diastereomeric with the other pair.
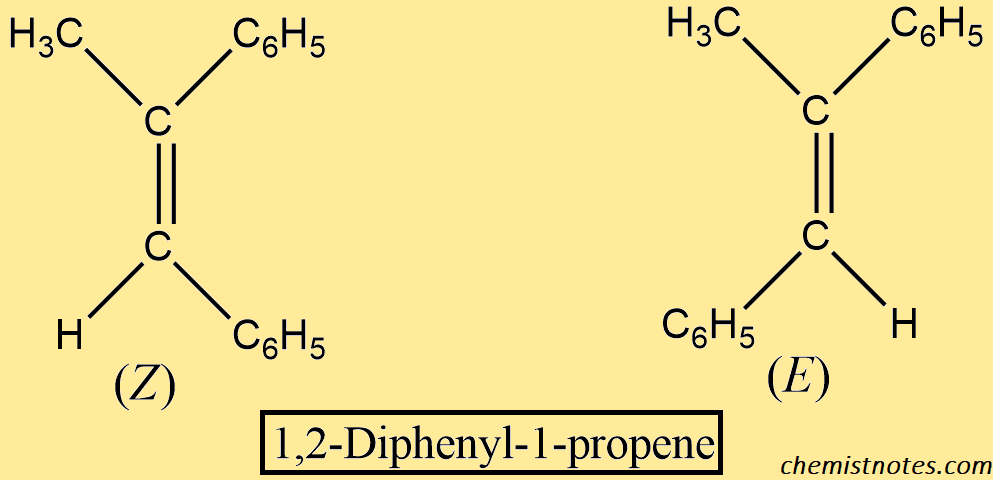
The product, too, exists as stereoisomers; a pair of geometric isomers, Z and E.
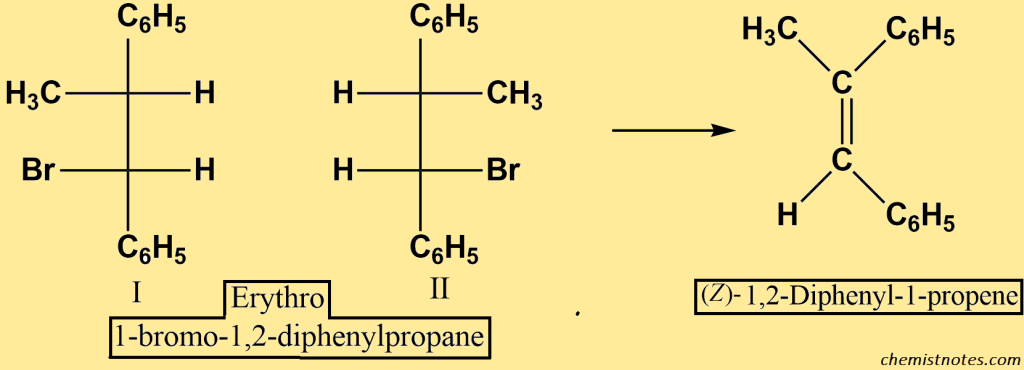
Now, if we start with the erythro halides I and II, we only contain the z alkene.
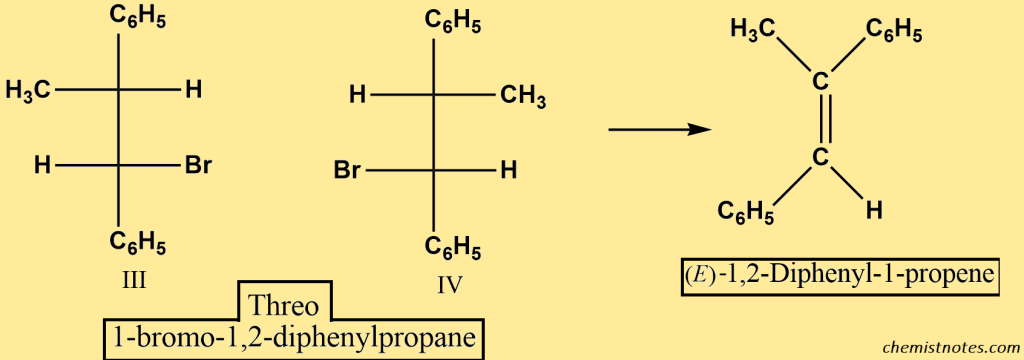
If we start with the threo halide, III and IV, we only obtain the E alkene.
Other studies have shown that results are typical: E2 elimination is both stereoselective and stereospecific.
Stereoselectivity vs. stereospecific
- Many reactions are like the addition of bromine, both stereoselective and stereospecific. But this is not always true. Some reactions are stereoselective but not stereospecific: one particular stereoisomer is the predominant product regardless of the stereochemistry of the reactant, or regardless of whether the reactant even exists as a stereoisomer.
- Some reactions are stereospecific but not stereoselective. Stereoisomers may react at widely different rates, but yield the same stereoisomers as the product or yield products that differ in ways other than in their stereoisomers reacts readily, and another does not react at all, as in the biological reactions we have referred to.
- The quality of stereoselectivity is concerned solely with the products, and their stereochemistry of a number of possible stereoisomeric products, the reaction selects one or two to be formed.
- The quality of stereospecificity is focused on the reactants and their stereochemistry; it is concerned with the products, too, but only as they provide evidence of a difference in behavior between reactants. Of steroisomeric reactants, each behaves in its own specific way.
- The stereospecificity of a biological reaction has given a powerful impetus to the development of synthetic methods that are highly stereoselective, In synthesizing a drug, for example, or a hormone, a chemist wants to use (stereoselective) reactions that produce just the correct stereoisomers, since only that stereoisomer will show (sterospecific) activity in a biological system.






