Table of Contents
ToggleHughes and Ingold proposed the E2 elimination reaction for the reaction that proceeds by second-order kinetics. The rate of reaction depends on the concentration of both alkyl halide and base used, such elimination reaction is of second-order kinetics and is known as an elimination bimolecular reaction. The loss of the leaving group is simultaneous with the removal of the proton by the base in E2 elimination.
Mechanism of E2 reaction mechanism
E2 (bimolecular elimination) reaction involves a single step:
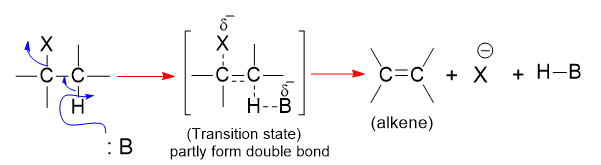
In this reaction mechanism, the base pulls a proton away from carbon, a halide ion departs, and a double bond forms. Halogen leaves an electron pair with it, hydrogen leaves an electron pair behind to form a double bond. The reaction undergoes a transition state.
In a transition state, there is a partially formed and broken bond. The energy necessary for bond breaking comes from bond formation between proton and base and π bond.
Kinetics of E2 elimination reaction
The rate-determining step is the only step that involves a reaction between a molecule of alkyl halide and a molecule of the base.
Rate = K [RX] [OH]–
Evidence for the E2 mechanism
- E2 reaction follows second order kinetics
- E2 reaction are not accompained by rearrangement
- show a large hydrogen isotope effect.
- are not accompained by hydrogen exchange; and
- show a lage element effect.
Orientation and reactivity of the E2 reaction
In E2 dehydrohalogenation the order of reactivity of alkyl halide is the same as the E1 reaction mechanism:
3o > 2o > 1o
Dehydrohalogenation reaction yields sometimes a mixture of isomeric alkenes. In such a case, it is necessary to identify which isomer predominates. It is possible to predict which isomer predominates on the basis of a molecular structure called the orientation of elimination.
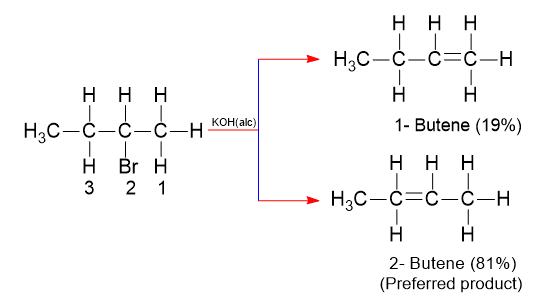
In dehydrohalogenation, the more stable the alkene faster it is formed. The predominant form of the more stable isomer is called Saytzeff’s orientation. In the E2 mechanism, there is a transition state
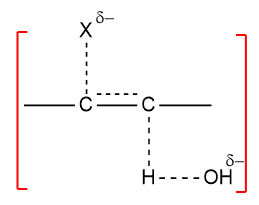
In the transition state, there is considerable alkene character. The factor that stabilizes the alkene (i.e. alkyl groups) also stabilizes incipient alkene in the transition state, Energy of activation is lowered and the alkene is formed faster. The product character transition state is a major factor to determine the stability of the product. The stability of alkene not only determines the orientation but also determines the reactivity of alkyl halide towards elimination.
Difference between E1 and E2
- March, J. Advanced Organic Chemistry, Reactions, 5th Edition; McGraw-Hill: New work, 1985.
- Indinavir synthesis: I. W. Davies and P. J. Reider, Chemistry and Industry (London), 1996, 412–15.
- G. Casiraghi, G. Casnati, G. Puglia, and G. Terenghi, J. Chem. Soc., Perkin Trans. 1,1980, 1862–65.






