Table of Contents
ToggleIsotopic dilution method is an analytical technique that is used to identify a single substance, let’s say A, that is present in a mixture of chemically related compounds (A, B, or C) from which A can not be quantitatively separated. With this technique, only a little amount of A needs to be isolated, and significant losses during its purification are acceptable.
It is one of the methods of optical purity ( percent enantiomeric excess) that is applied for the resolution of a racemic mixture.
Isotopic dilution method
This method is one of the reliable methods for determining the optical purity or enantiomer excess (ee) of a sample. In this method, a known weight of the sample under examination is taken and it is mixed with a known weight of isotopically labeled racemic modification of the same compound of known enantiomer composition in solution. That is, this method involves the dilution of at least one of the enantiomers in the sample. The racemic form is separated by the crystallization process.
Principle of Isotopic dilution method
As explained above, the basic principle behind this method in the context of enantiomeric purity is that a substance with an unknown enantiomer composition is diluted by the corresponding isotopically labeled substance with a known enantiomer composition. Determining a dilution factor that can be correlated to the enantiomer composition of the unknown mixture requires measuring the isotope concentration of the labeled and unlabeled samples and isolating either a pure enantiomer or a pure racemate.
It causes the labeled racemic form to be isolated again. In solution, the racemic substance separates into molecules with (+) and (-) labels. One of the enantiomers is isotopically diluted in the recovered dl pair if one of the labeled molecules mixes with the enantiomer molecule whose optical purity needs to be determined while the other is unaffected. However, all the molecules in the recovered dl pair will be diluted if one mixes with another or with its opposite enantiomer in unlabelled racemic material. Thus, a chemically pure enantiomer or pure racemate is obtained throughout this process.
By the measurement of the isotopic concentration in the labeled and unlabelled samples and reisolation of pure enantiomer or pure racemate, the optical purity of the original sample can be calculated. Furthermore, knowing the weights of the original active material and the newly added labeled racemic material allows one to determine a dilution factor and show how active the recovered labeled racemic material will be. Then, the experimental activity is compared with the expected activity. If the experimental activity is lower than expected, there may have been some trace amounts of racemic material (unlabelled) in the reportedly pure enantiomer.
Determination of Optical Purity
On considering a sample to be a mixture of enantiomer and corresponding racemic modification, the relation between the fraction of isotope label before and after reisolation, assuming that there is no
isotope effect during the crystallization is given by the equation:
xCo = (x+y)c
Where x and y are the amounts of the isotope-labeled racemate (or other mixture of known enantiomer composition) and of substance to be determined, respectively. The parameter Co and C are the specific radioactivity of isotoped labeled racemic substance and new specific radioactivity after resolution by recrystallization.
The recovered racemic material is given by:
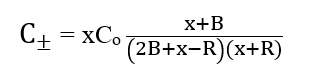
Where B = weight of resolved material whose purity is to determine admixed with x and R = weight of racemic substance if any present in B.
Now solving the equation for R, the optical purity can be given as follow:
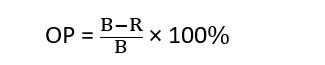
Application of Isotope dilution method
- Determination of enantiomer excess.
- Applicable for determining the mass and quantity of chemical substances.
- In the determination of optical purity.
- In the determination of the geological age of minerals.
- For the analysis of the unknown radioactive samples.
Isotopic dilution method video
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






