Table of Contents
TogglePhosphorus ylides are, commonly known as Wittig reagents, found to be used in a wide variety of reactions, especially in synthetic chemistry for the synthesis of naturally occurring compounds as well as compounds with biological and pharmacological activity.
These compounds were synthesized for the first time more than 100 years ago, at the end of the nineteenth century, by Michaelis and co-workers. The discovery of phosphorus ylide led to the development of a new method for the synthesis of alkenes, which is universally known as the Wittig reaction and it has found widespread application in synthetic organic chemistry as mentioned earlier.
What is phosphorus ylide?
Phosphorus ylides are defined as compounds in which carbon is directly bonded to a positively charged phosphorus atom. These compounds have gained much importance as extensively used reagents for joining synthetic building blocks through the creation of carbon-carbon double bonds.
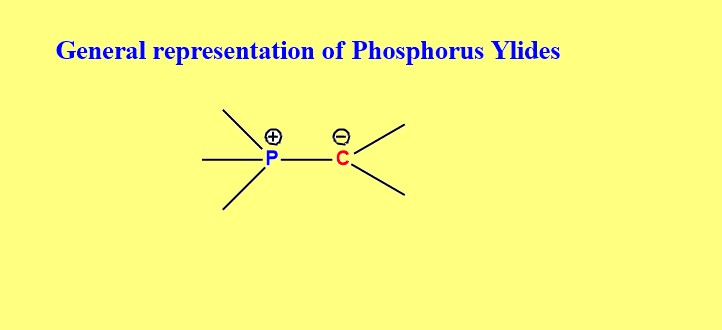
Moreover, phosphorus ylides can be represented by two canonical forms viz. ylene and ylide, as shown below.
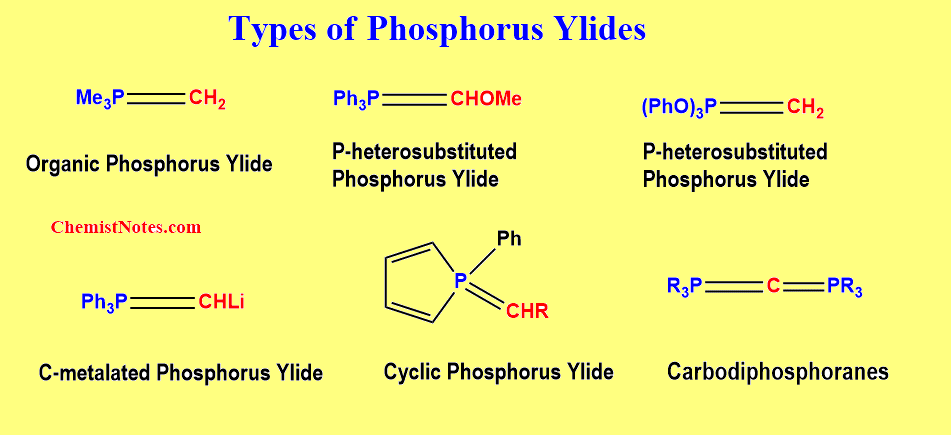
Types of Phosphorus ylide
Various classes of these chemicals have now been synthesized. Phosphorus ylides are classified as stabilized, semi–stabilized, or non-stabilized in the chemical literature, based on the delocalization of the negative charge on the ylidic carbon atom by substituents.
But, classifying these compounds according to the type of atoms or groups associated with the phosphorous and carbon atoms of the P=C bond is perfectly reasonable. On this basis, phosphorus ylides are of the following types.
- Organic phosphorus ylide
- C-heterosubstituted phosphorus ylide
- P-heterosubstituted phosphorus ylide
- C-metallated phosphorus ylide
- Cyclic phosphorus ylide
- Carbodiphosphoranes and phosphacumuleneylids
Phosphorus ylide preparation
The various methods available for the synthesis of phosphorus ylides are described below.
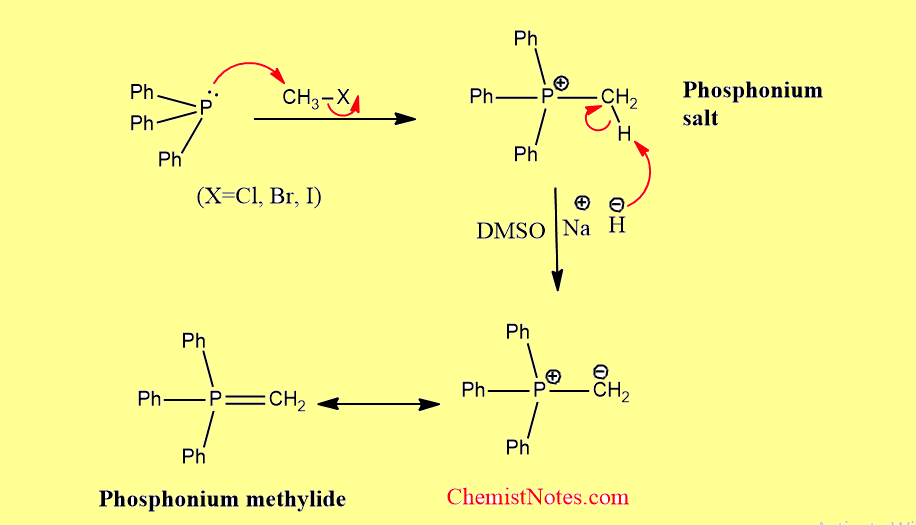
From phosphonium salts
This is the most general method of preparation which involves two steps: the formation of phosphonium salts and, the deprotonation of salts to the ylide. Generally, salt is deprotonated by using a suitable base.
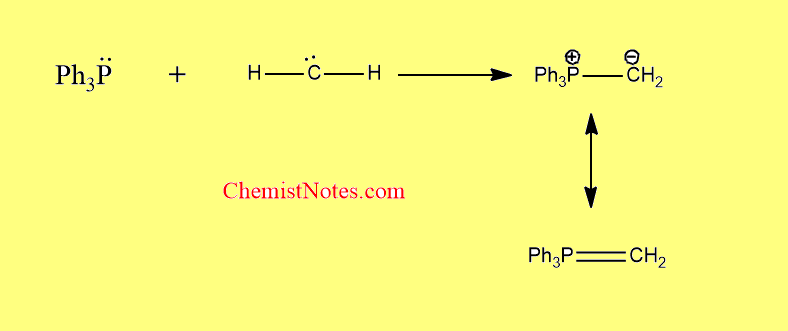
From carbene
The suitable carbene undergoes addition to a phosphine to form phosphorus ylides.
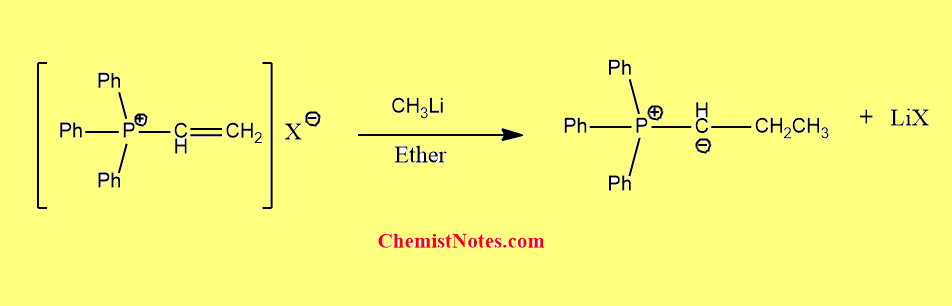
From vinyl onium salts
When vinyl onium salts react with suitable carbanion( For example, methyl lithium) in presence of ether, ylides are formed.
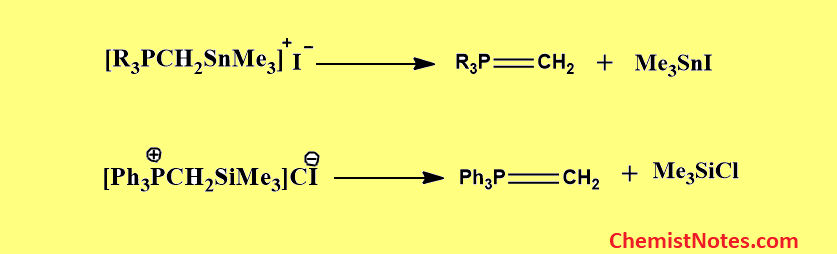
From α-silyl- and α-stannyl-substituted phosphonium salts
When trimethyl tin phosphonium iodides are heated, iodotrimethyltin is expelled and the appropriate phosphorus ylides are formed.
Preparation of phosphonate carbanion
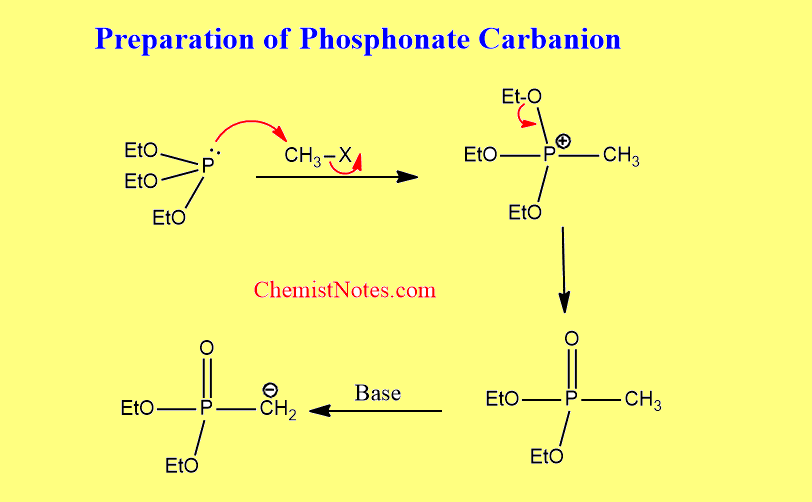
Phosphorus ylide reaction
Phosphorus ylides undergo a wide range of reactions due to their distinct molecular and electronic structure. Some of the general reactions are shown below.
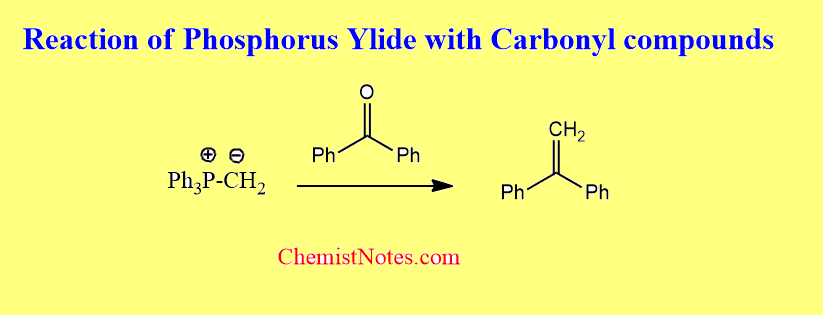
Reaction with carbonyl compounds
Phosphorus ylides are commonly known as Wittig reagents, which react with aldehydes or ketones to form an alkene product. This reaction is called the Wittig olefination reaction.
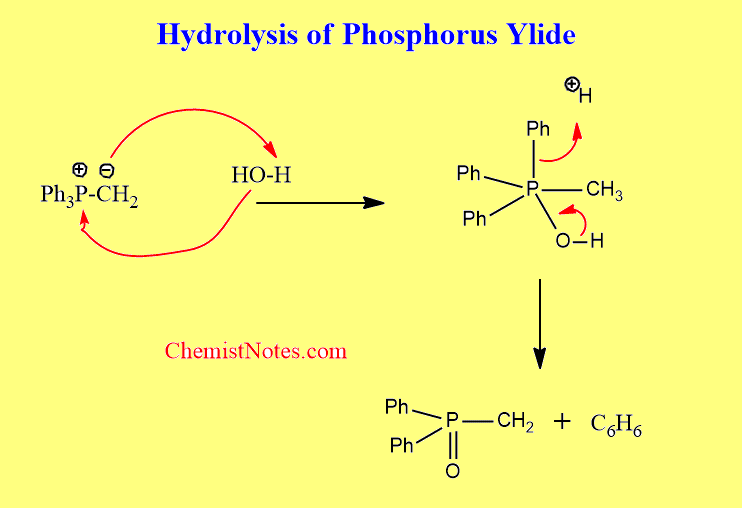
Hydrolysis of ylides
Hydrolysis of phosphorus ylides results in cleavage of the carbon-phosphorus multiple bonds. The ylidic carbon atom is converted to a methyl group producing hydrocarbons, whereas the phosphorus becomes a P=O.
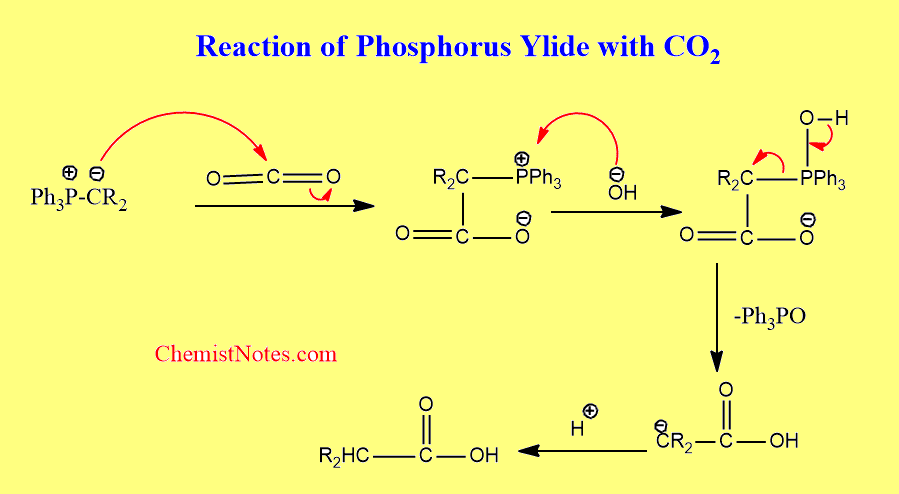
Reaction with CO2
The ylide reacts with carbon dioxide in presence of a base to form carboxylic acids.
Application of phosphorus Ylides
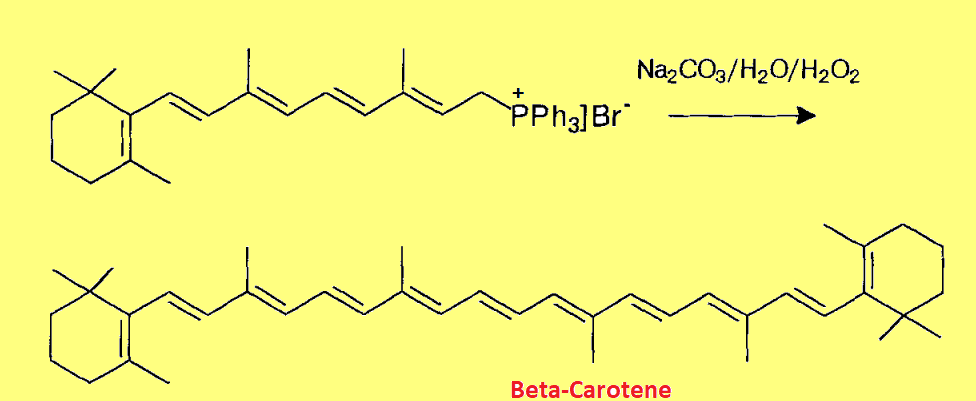
These are very important organic reagents, used for the preparation of olefins, natural products, and many other synthetic compounds. For example; β-carotene is also prepared by using phosphorus ylides.