Table of Contents
ToggleRubbing alcohol, also known as surgical spirit or isopropyl alcohol, is a very potent solvent that can easily remove even the most obstinate oil and filth.
Either isopropyl alcohol or ethanol-based liquids can be used as rubbing alcohol, with isopropyl alcohol products being the most widely available. However, only 70% or 99% isopropyl alcohol concentrations of rubbing alcohol are available for purchase. Although you would expect the higher concentration would be more effective, disinfection analysts claim that 70% is actually preferred. Because it contains more water, it dissolves more slowly, enters cells, and kills bacteria. At concentrations greater than 80%–85%, rubbing alcohol loses some of its disinfectant properties.
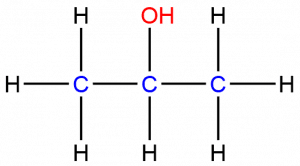
The rubbing alcohol structure can be illustrated as:
History behind the name: rubbing
Midway through the 1920s, the term “rubbing alcohol” became well-known in North America. Because it was originally used as a liniment for massage, rubbing alcohol got its name. In some formulations, this original rubbing alcohol was perfumed and contained different additives, most notably a higher concentration of methyl salicylate. This original rubbing alcohol was quite different from today’s precisely formulated surgical spirit.
Myth about rubbing alcohol
Rubbing alcohol can really be highly harmful, especially for young children, and is NOT TRUE for lowering fevers. This was a prevalent fallacy that used to be disproven.
Rubbing alcohol and its Background
The simplest example of secondary alcohol is isopropyl alcohol, in which the alcohol carbon is joined to two additional carbons. Propanol’s structural isomer is that substance. In an ultraviolet-visible spectrum, isopropyl alcohol has a maximum absorption at 204 nm. It is frequently used as a solvent and cleaning agent, especially for removing pollutants that are lipophilic, like oil.
What % of isopropyl alcohol is used in rubbing alcohol?
Contrarily, rubbing alcohol also includes additional elements like water in addition to isopropyl alcohol. 70% isopropyl alcohol is present in most brands of rubbing alcohol.
Application of rubbing alcohol
A typical household chemical is rubbing alcohol. It offers a range of potential applications for both daily household cleaning and personal care. Some of the major uses of rubbing alcohol are:
- Remove ink stains: Apply hand sanitizer, hairspray, or rubbing alcohol on the stain to thin it down and make it simpler to wash out. The majority of ink stains may be removed with these solvents.
- Deodorising shoes: To get rid of the odor, apply to rub alcohol to the portion of your shoes that is soiled or worn. Rubbing alcohol not only works as a natural deodorizer for shoes, but it also helps to clean and disinfect the shoe.
- Disinfecting tick bites: After it is removed, they can clean the bite with rubbing alcohol. The place where the tick had adhered itself should be cleaned with rubbing alcohol applied to a cotton swab.
- Make a DIY homemade ice pack
- Can ease an ear infections
- Get rids of plant pests
- Eases nausea after surgery
- Cleaning cuts
- Hair dye
- Cleaning up around the house
- Cleaning jewelry
Harmful risk of using rubbing alcohol
If isopropyl alcohol is ingested, the person may appear intoxicated. Sedation, slurred speech, lethargy, and vomiting are side effects. Additionally, isopropyl alcohol severely irritates the digestive system. It can make you feel sick, make you have diarrhea, hurt your stomach and intestines, and even make you bleed. Dehydration, low blood pressure, shock, and coma are all possible outcomes.
Difference between rubbing alcohol and isopropyl alcohol
The concentration is the critical difference between isopropyl alcohol and rubbing alcohol. Isopropyl alcohol has a 100% concentration, whereas rubbing alcohol has a lower concentration due to the addition of water. The characteristics of the two liquids are identical otherwise.
What % of isopropyl alcohol is used in rubbing alcohol?
Contrarily, rubbing alcohol also includes additional elements like water in addition to isopropyl alcohol. 70% isopropyl alcohol is present in most brands of rubbing alcohol.
Interesting queries related to rubbing alcohol
1. Can rubbing alcohol be used to make hand sanitizer?
Only ethanol (ethyl alcohol) or isopropyl alcohol may be used as active components in alcohol-based hand sanitizers (isopropanol or 2-propanol). On hand sanitizer labels, the word “alcohol,” when used alone, expressly refers to ethanol alone.
2. Is rubbing alcohol good for skin?
Rubbing alcohol and hydrogen peroxide should not be used to open wounds or used to treat acne or greasy skin. They are ineffective, and they can harm your skin, exacerbating the issue. To treat acne, use an over-the-counter product with salicylic acid or benzoyl peroxide. To clean a wound, only use soap and water.
3. Is rubbing alcohol used to cure acne?
Rubbing alcohol can somewhat reduce the appearance of pimples, but because of its negative side effects and lack of scientific support, this treatment isn’t recommended for long-term use. One of the various Home treatments for acne that are discussed online is rubbing alcohol. Understanding the science behind rubbing alcohol is crucial before you reach for it in your medicine cabinet.
Alcohol is known officially as isopropyl. It is commonly accessible and reasonably priced at your neighborhood drugstore, typically in the first aid section. 70% of OTC rubbing alcohol is isopropyl; the remaining 30% is water or oil. But it is not mentioned that rubbing alcohol alone can be used to treat acne but can be effective along with benzoyl peroxide and other medications.
Rubbing alcohol Video
FAQs
Can rubbing alcohol be used for whitening teeth?
Ans: Rubbing alcohol can’t be used for whitening teeth.
Can rubbing alcohol be used to clean pierced ears?
Rubbing alcohol can’t be used to clean pierced ears, it can be dangerous.






