Table of Contents
ToggleVictor Meyer’s method is one of the most applied methods for the identification of alcohols. In this method, the colour of the resulting solution is observed after the unknown alcohol is submitted to a series of chemical analysis.
Victor Meyer’s Method to Identify Alcohol
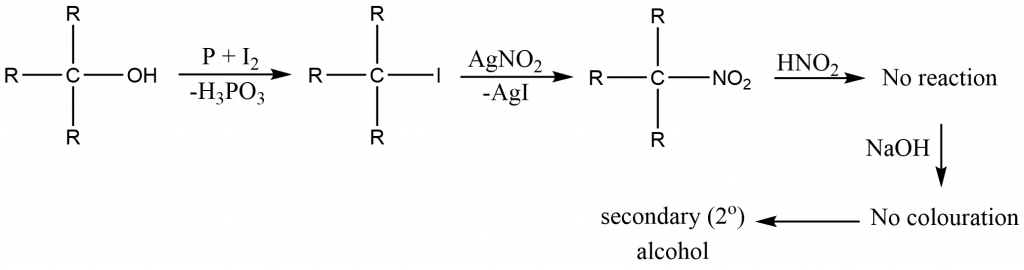
When alcohol is treated with phosphorus and iodine, an alkyl halide is formed, which is subsequently treated with silver nitrate to produce the nitroalkane. The nitrous acid (NaNO2 + HCl) is then added, followed by the addition of strong alkalies such as NaOH or KOH to make the solution alkaline. Now, the color of the solution is observed carefully in order to distinguish primary, secondary, and tertiary alcohol.
- Blood red colour confirms the presence of primary alcohol
- Blue colour confirms the presence of secondary alcohol
- A colourless solution identifies tertiary alcohol
Victor Meyer’s test for alcohol
Victor Meyer’s test is performed as described above, and the involved reactions can be presented as:
For primary alcohol

For secondary alcohol

For tertiary alcohol
Victor Meyer’s method Video
References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.






