Table of Contents
ToggleA lot of information can be obtained from knowledge of the molecular formula of an unknown substance. One such information is an index of hydrogen deficiency, which is very useful in structural determination problems. This is also known as Ring Double Bond Equivalent (RDBE). This concept is mainly used in Mass analysis in which the molecular formula is determined first using relative intensities value and m/z value from the mass spectrum, then using obtained molecular formula, we can determine possible structures of the given compounds.
What is index of hydrogen deficiency?
The hydrogen deficiency index is an important concept used in structural elucidation in order to determine the degree of unsaturation of molecules. One important point is that deviation from an acyclic alkane molecular formula or saturated formula CnH2n+2 takes place when there is a presence of either π-bonds or rings.
The index of hydrogen deficiency (HDI) which is also known as the unsaturation index is the number of π– bonds and/ or rings a molecule contains. In another word, it is the number of pairs of hydrogen atoms that must be removed from the corresponding saturated formula to produce the molecular formula of the compound of interest.
The value of the hydrogen deficiency index helps to predict the structure of compounds. It means if you have a molecular formula of unknown compounds, first determine the value of the hydrogen deficiency index and the obtained value of HDI plays important role in determining the correct structure of the unknown compounds.
- A compound with an index of one must have one double bond or one ring but it can not have both structural features.
- A compound with an index of two could have a triple bond or it could have two double bonds or two rings or one of each.
- Any substance with an index of four or more may contain a benzenoid ring, a substance with an index less than four can not contain such a ring.
How to find index of hydrogen deficiency
To determine the index of hydrogen deficiency for a compound, apply the following steps.
Step 1: Determine the formula for the saturated acyclic hydrocarbon containing the same number of carbon atoms as the unknown substance.
Let an unknown substance has the molecular formula C7H14O2. Now, determining the general formula for a saturated acyclic hydrocarbon( CnH2n+2). Here, n=7 thus, the formula becomes= C7H16
Step 2: Correct this formula for the nonhydrocarbon elements present in the unknown substance. Add one hydrogen atom for each Group V element present and subtract one hydrogen atom for each group VII element present.
There are two oxygen atoms in the formula of unknown. Therefore, adding two oxygen atoms and the corrected formula becomes= C7H16O2.
Step 3: Compare this formula with the molecular formula of the unknown. Determine the number of hydrogens by which the two formulas differ.
Corrected formula= C7H16O2
Unknown formula= C7H14O2
The difference in the number of hydrogen between these formula= 2
Step 4: Divide the difference in the number of the hydrogen atom by 2 to obtain the index of hydrogen deficiency.
Index of hydrogen deficiency= (Difference in no. of hydrogen atoms)/ 2= 2/2=1
The index of hydrogen deficiency equals one. There must be one ring or one double bond in the unknown substance.
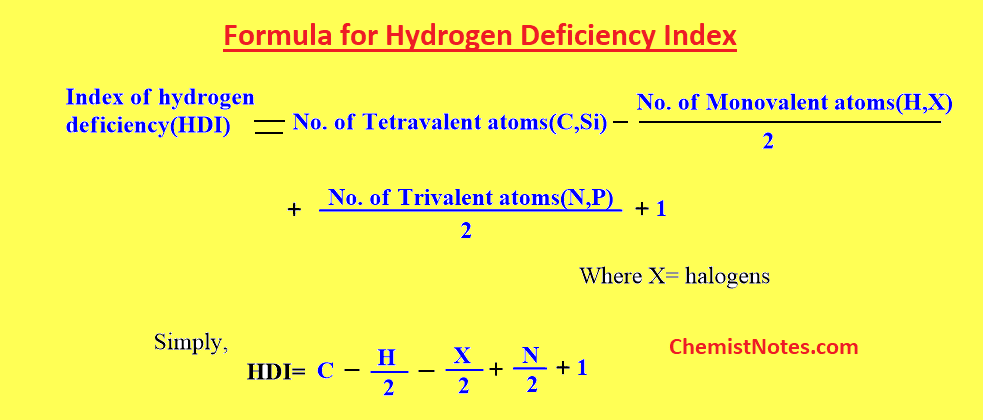
Index of hydrogen deficiency formula
The index of hydrogen deficiency can be calculated, alternatively, for compounds containing carbon, hydrogen, nitrogen, halogen, oxygen, and sulfur by using the following formula.
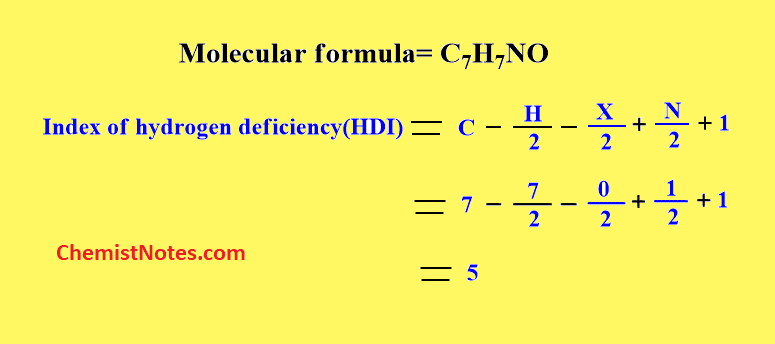
Let’s have an example of the calculation of HDI. For example, we have molecular formula C7H7NO calculating index hydrogen deficiency.
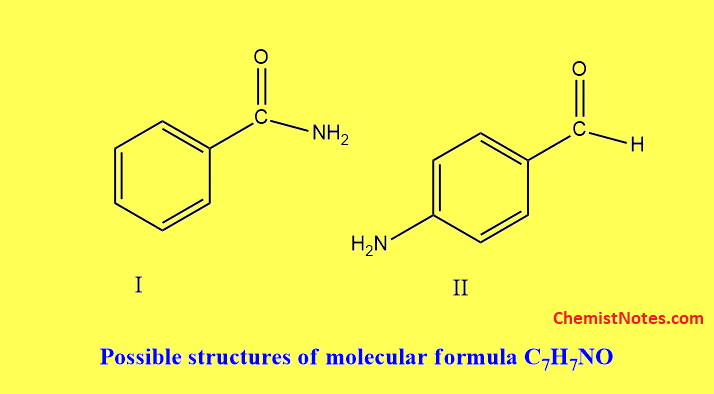
HDI value 4 or more generally indicates the presence of a Benzene ring. Thus, the possible structures of the compounds are shown below:
Calculate the index of hydrogen deficiency for the following molecule or compounds
- Benzene
- Benzaldehyde
- Picric acid
- Naphthalene
- Anthracene
- Benzophenone
- DMSO(Dimethyl sulfoxide)
- Phenol
- Benzoic acid
- Benzamide
FAQs/MCQs:
What is an index of hydrogen deficiency?
The index of hydrogen of a compound is the number of π- bonds and/ or rings a molecule contains.
What does a hydrogen index deficiency tell us?
The hydrogen index deficiency tells us about the number of π- bonds and/ or rings present in the given compound.
What is the index of hydrogen deficiency of cyclohexene?
The index of hydrogen deficiency of cyclohexene is 2.






