Table of Contents
ToggleWhen γ,δ- unsaturated carboxylic acid is treated with iodine in presence of sodium bicarbonate, a lactone is formed. This reaction is known as iodolactonization. This reaction is also known as iodocyclization or halolactonization.
Iodolactonization: General introduction
The conversion of γ,δ-unsaturated carboxylic acid derivatives into a five- or six-membered lactone when an iodine source like I2+ NaHCO3+H2O or KI+I2+NaHCO3 is present. This reaction is commonly known as iodolactonization. A similar reaction with a bromine source is called bromolactonization.
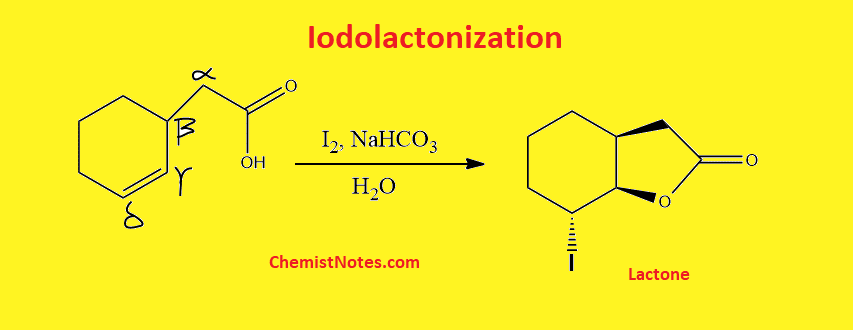
This reaction has been used for the synthesis of various natural products such as vernomenin etc.
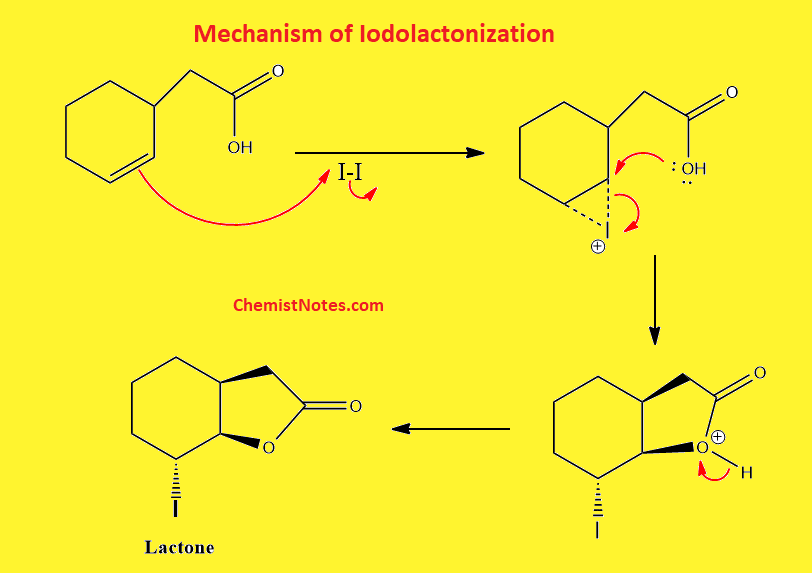
Iodolactonization mechanism
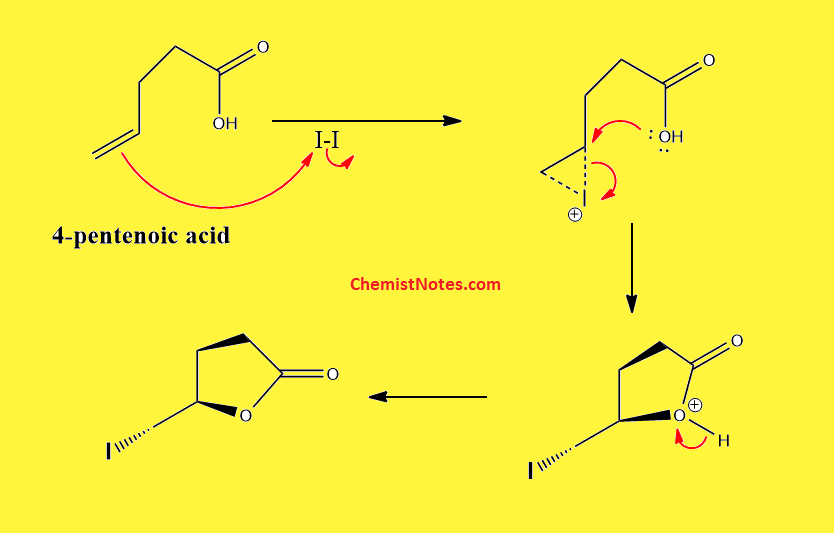
First of all, an unsaturated double bond attacks the iodine forming the iodonium ion. This iodonium ion is then opened by the carboxylate ion. The detailed mechanism of this reaction is shown below:
Iodolactonization of 4-pentenoic acid
When 4-pentenoic acid undergoes a reaction with iodine and sodium bicarbonate, the following structure is formed.