Table of Contents
ToggleKnoevenagel condensation is a reaction between aldehyde or ketone and activated methylene compound in presence of a base catalyst. This reaction was first of all reported by Emil Knoevenagel in 1894.
Knoevenagel condensation reaction
Knoevenagel condensation is a nucleophilic addition of a compound with an active methylene component to a ketone or aldehyde followed by the elimination of water to form an olefin. This reaction is considered the extension of aldol addition and it is reversible in nature.
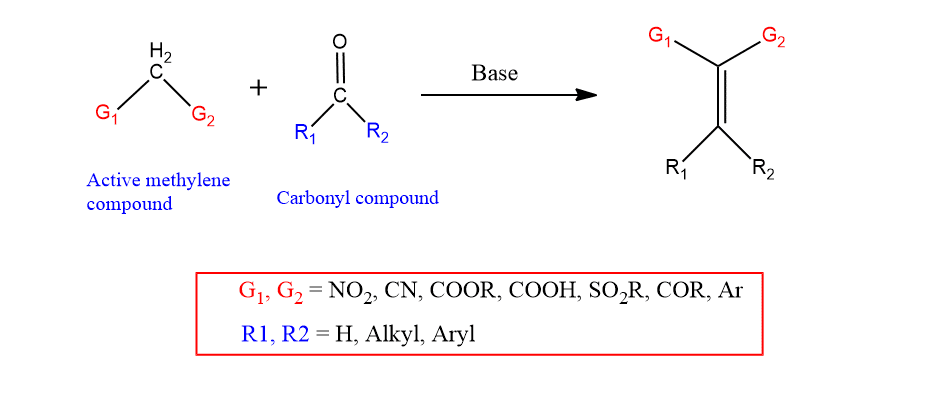
At least one electron-withdrawing group, such as nitro, cyano, carbonyl, or sulfonyl, can activate the methylene group. Thus, any of these electron-withdrawing groups close to a methylene group can act as a nucleophile. For example, malonic acid, malonic ester, Meldrum’s acid, β-ketoacid, and β-ketoester can be used as nucleophiles in this reaction.
Weak bases such as piperidine, diethylamine, pyridine, amino acids, and others are commonly used to catalyze this reaction.
Knoevenagel condensation mechanism
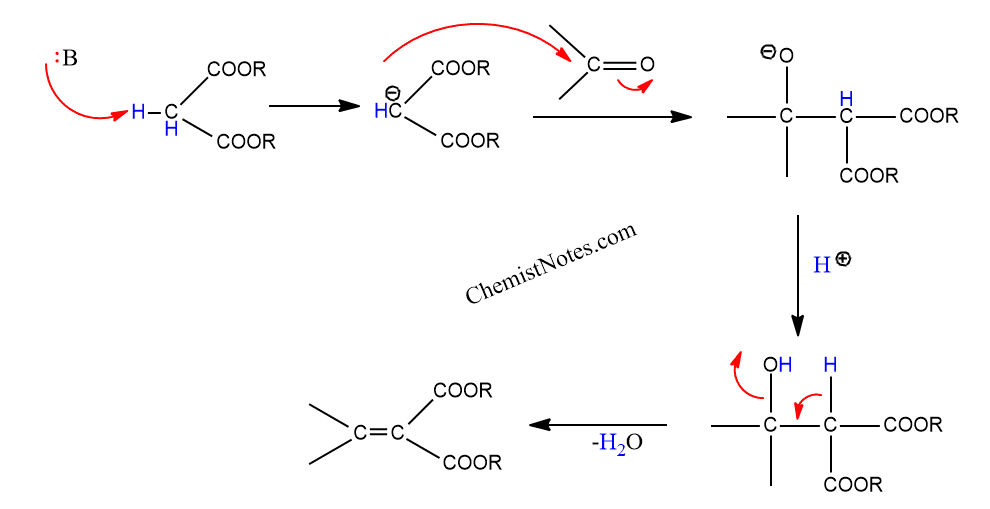
The mechanism of knoevenagel condensation involves the following sequential steps, as discussed below:
The deprotonation of the acidic hydrogen from the methylene molecule is the first step. Organic bases, such as amines, can be utilized for deprotonation. The corresponding anions generated by deprotonation combine with the carbonyl substrate to form the aldol type intermediate. The loss of water from the intermediate results in a primary α,β-unsaturated condensation product.
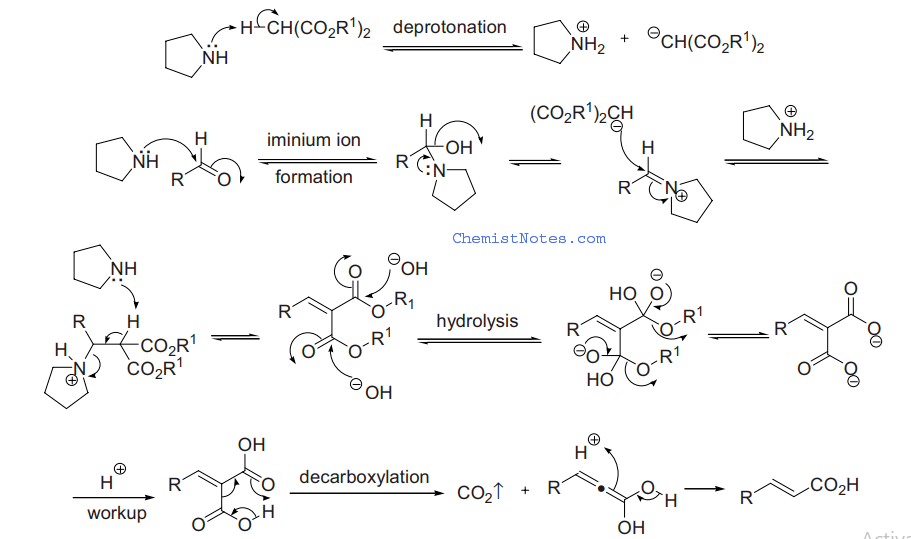
There is another mechanism based on knoevenagel’s findings that is validated by more recent research. This mechanism involves the formation of an intermediate iminium species.
Knoevenagel condensation application
This reaction has been used to make coumarin, indole, quinoline, stilbenes, and other intriguing compounds.







One Response
that’s awesome explanation