Table of Contents
ToggleChromatography is an analytical technique to separate the components of a mixture which are distributed between a stationary phase and a flowing mobile phase according to the rate at which these components are transported through the stationary phase.
Chromatography is an analytical technique that is used for the separation, purification, isolation, and identification of chemicals components present in a mixture. It is one of the important tools for chemists, biologists, and many related fields.
Chromatography consists of two phases:
- Stationary phase: The phase over which the mobile phase flows is called the stationary phase. Stationary phase is generally made up of silica gel, alumina, charcoal, starch, aluminum silicate, activated alumina etc. Stationary phase is packed in a glass plate or tube.
- Mobile phase: The phase which flows over the stationary phase is called the mobile phase. Mobile phase is generally liquids or mixtures of liquids or gases which carries components to be separated.
Types of Chromatography
The chromatography can be classified into different types on the basis of different criteria.
A. The chromatography is classified on the basis of the physical state of the mobile phase and stationary phase
- Classification on the basis of stationary phase
- Adsorption chromatography: Here stationary phase is solid.
- Partition chromatography: Here, Stationary phase is liquid.
- Classification on the basis of mobile phase
- Liquid chromatography: Here, mobile phase is liquid.
- Gas chromatography: Here, mobile phase is gas.
B. Classification on the basis of Shape of Stationary phase/On the basis of chromatographic Bed shape.
- Column chromatography: Here, the stationary phase is in the form of column and placed inside the tube.
- Planer chromatography: Here, the stationary phase is in the form of a planar shape.
C. Classification on the basis of mechanism of separation
- Size Exclusion Chromatography: Here, separation of different particles is based on the molecular size and shape of particles present in the sample.
- Ion Exchange Chromatography: Here, the separation of ions and polar molecules is based on their affinity to the ion exchanger.
These are further classified into various types as shown in the figure.
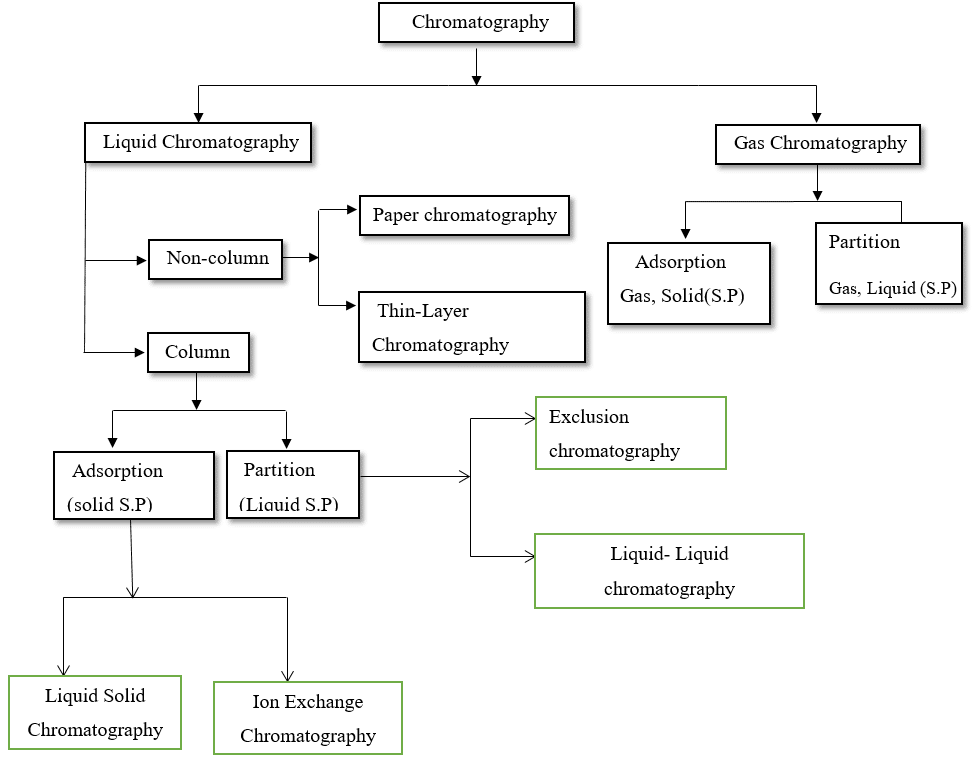
Most commonly employed chromatographic techniques
The most commonly used chromatographic techniques are listed below:
- Column Chromatography
- Ion-Exchange Chromatography
- Affinity Chromatography
- Exclusion Chromatography
- Paper Chromatography
- Thin Layer Chromatography
- Gas Chromatography
- High Performance Liquid Chromatography
How does Chromatography works( Principle of Chromatography)
The chromatography is consisting of the mobile phase and stationary phase. The mixture of components to be separated and solvents together constitute the mobile phase. When the mobile phase flows over the stationary phase different types of interaction or phenomena occur between particles of the stationary phase and that of the mobile phase. The separation of components of mixtures depends on different types of phenomena such as solubility, adsorption etc. The solubility of components to be separated highly depends on the polarity of solvents used. So, if one of the components of a mixture is soluble in the solvents used, it is carried by the solvent at a rate equal to the flow of solvents but the insoluble components move slower in the stationary phase. In this way, the components are distributed in two phases and their migration rate becomes different and separation occurs.
Similarly, if one of the components of mixtures has a high affinity for the stationary phase and interacts with the stationary phase, and gets adsorbed, it moves slowly. As we know different solutes or components of the mixture have a different affinity of stationary phase, their rate of migration becomes different and hence separation occurs.
Application/uses/purpose of chromatography:
Chromatography is a widely used analytical technique in various fields such as Pharmaceuticals, Food and beverage industries, Chemical industries, Forensic science, Environmental analysis, etc.
- Chromatography is mainly used in the separation of components of mixture.(Separation)
- It is used to check the purity of compounds( purification check)
- To check if the given two compounds are identical(identification)
- To monitor the progress/rate of a reaction.
FAQs
What is retention factor(migration parameter)?
Retention factor is defined as the ratio of the distance travelled by the solute from the original line and the distance travelled by the solvent from the original line. It is denoted by Rf .
Rf’=(Distance travelled by the solute)/(Distance travelled by the solution)
What are the factors that affect the value of Rf?
The value of the Retention factor depends on the nature of solvents used, nature of solutes or substance used, temperature, and porosity grade of the filter paper.






