Table of Contents
ToggleFractional distillation is the process of separation of chemical mixtures employing heat to separate the components present in the mixture. It is one of the popular separation methods in chemistry experiments.
Principle of fractional distillation
The basic principle of fractional distillation is that different liquids boil at different temperatures. When the mixture is heated, the liquid with a lower boiling point boils faster and converts into vapor before the other. Thus, the liquid with a low boiling point escapes from the mixture and hence, the component gets separated. It is the boiling point from which we can determine what given compound is separated from the mixture. For example; the two miscible liquids viz acetone and water can be separated by applying different temperatures i.e. escaping of liquid at the temperature of 560C identifies the separation of acetone from the mixtures.
Conditions for fractional distillation
- The mixture of liquids must be miscible with each other.
- The miscible solvents/liquids must have a different boiling point.
Process of fractional distillation(Working process)
The process involves the heating of the liquid mixture and separation. Assume the liquid mixture is made up of two liquids (A and B), with B having a slightly higher boiling point. When the liquid mixture is heated to boiling, the vapors of A and B rise through the fractionating column. Since A is more volatile, its vapor starts rising in the flask. These vapors from volatile components of A then pass through the condenser from the fractionating column and are collected as distillate in tubes or beakers. The process is followed by repeated vaporization and condensation for the complete separation of two miscible liquids.
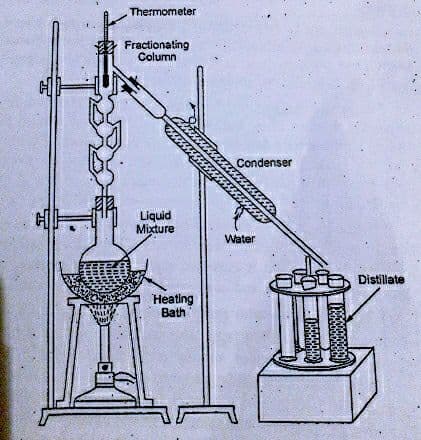
Fractional distillation column (Fractionating column)
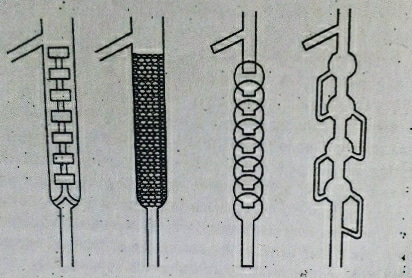
Fractionating column, fitted to the distillation flask at its mouth is a long column that provides a large surface area to obstruct the free passage of the vapors upwards and that of liquids downwards. The bottom of the column is hot, while the top is cool. High boiling point substances condense at the bottom, while low boiling point substances condense on their way to the top. Fractionating columns aid in the separation of the mixture by allowing the mixed vapors to cool, condense, and vaporize again according to Raoult’s law. There are varieties of fractionating columns available in the market. Some of them are shown below.
Example of fractional distillation
Separation of o-xylene from p-xylene by fractional distillation
The boiling point of o-xylene is 1440C and that of p-xylene is 138oC. The liquid mixture in the round bottom flask is heated over the sand bath and when the temperature reaches near the boiling point of p-xylene, it starts to vaporize. The vapors of p-xylene are then collected in the receiver after condensing.
Other examples of fractional distillation are
- Fractional distillation of ethanol and water
- Fractional distillation of Crude oil/Petroleum products
Advantages of Fractional distillation
The major advantages of this technique are listed below.
- This technique is used to separate two or more liquids that are miscible from each other
- This is used in several industries for the separation and purification of organic compounds.
- This method helps us to check the purity of components.
Simple distillation vs Fractional distillation
The major difference between simple distillation and fractional distillation is that simple distillation separates liquids mixture having large boiling point differences while fractional distillation separates liquids mixture having similar boiling points(not same but slightly different).
| Simple distillation | Fractional distillation |
|---|---|
| Simple distillation is used to separate the liquid from the mixture if the boiling points of liquids are sufficiently different i.e. at least 25°C. | Fractional distillation is used to separate the liquids from the mixture having similar boiling points. |
| Generally, it is used to separate liquids from nonvolatile solid impurities. | Generally, it is used to separate one liquid from a mixture of liquids. |
| The apparatus of this distillation does not have a fractioning column. | The apparatus of this distillation has a fractioning column. |
| In this technique, the vaporization-condensation process of a particular liquid takes place only one time. | In this technique, the vaporization-condensation process of a particular liquid takes place several times |
| It is less efficient than fractional distillation | It is more efficient than simple distillation |
| Apparatus setup is simpler | Apparatus setup is not simpler |
| It requires less time for separation. | It requires more time for separation. |
| It requires less energy to completion | it requires more energy to completion |
| Example: Purification of seawater, separation of aniline and nitrobenzene, etc | Examples: Purification of petroleum products, separation of crude oil, separation of ethanol from water, etc. |
FAQs
Why is fractional distillation more popular/efficient than simple distillation?
Since both evaporation and condensation, which ensures complete separation of volatile miscible liquids takes place simultaneously, separation of the liquid when the difference in their boiling point is less than 250C, and the greater efficiency due to the use of fractionating column takes place via fractional distillation, it is considered as one of the popular distillation techniques.
What is Fractional distillation?
Fractional distillation is the process of separation of chemical mixtures employing heat to separate the components present in the mixture.
What is the apparatus needed for fractional distillation?
The apparatus for fractional distillation consists of following
1. Distillation flask
2. Fractionating column
3. Condenser
4. Collector
In which condition, fractional distillation is used?
Fractional distillation is used when the two miscible liquids mixture has slightly different boiling points.






