Table of Contents
ToggleBorodine hunsdiecker reaction mechanism was first of all reported by Borodine in 1861 but later Hunsdiecker studied this reaction extensively and made this reaction practically possible. Silver carboxylates can be decarboxylated by treatment with bromine to yield alkyl bromide, this reaction is called hunsdiecker reaction.
Borodin hunsdiecker reaction
The Hunsdiecker reaction is the breakdown of carboxylic acid salts of Ag(I) in the presence of halogen into organic halides with one less carbon atom than the original acids. Generally, carbon tetrachloride is used as a solvent.
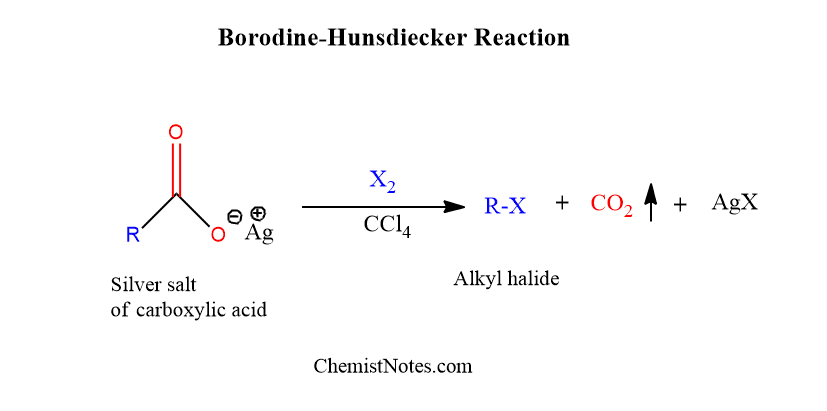
This reaction is also known as Borodine-Hundiecker reaction, hunsdiecker borodine reaction, hunsdiecker decarboxylation, hunsdiecker bromodecarboxylation and hunsdiecker degradation. Thus, in this reaction alkyl bromide or halide is formed from carboxylates.
Borodine hunsdiecker reaction mechanism
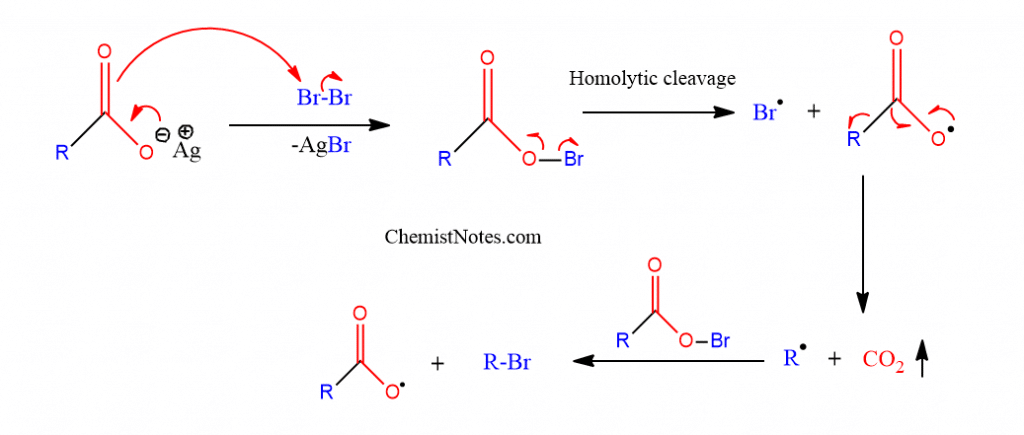
This reaction is most likely to happen via a radical chain mechanism, with carboxyl radicals forming as intermediates. Moreover, a free radical process involving the breakdown of intermediate hypohalite is supported by a number of experimental data.
Initially, bromine combines with silver carboxylate to form an acyl hypobromite species. By homolytic cleavage of the O-Br linkage, the acyl hypobromite decomposes into a Bromo radical and a carboxyl radical. The carboxyl radical further decomposes to carbon dioxide and an alkyl radical, which then interacts with hypobromite to produce alkyl bromide and additional carboxyl radicals.
Application of hunsdiecker reaction
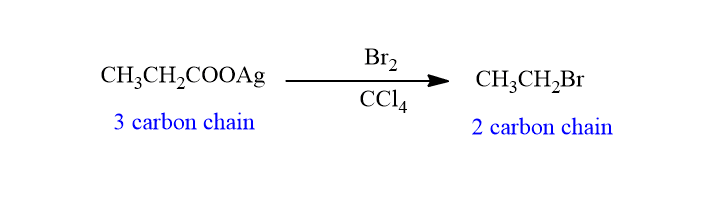
This reaction is widely used in the synthesis of aliphatic halides. Moreover, this reaction is used to decrease the number of carbon atoms in the chain.
Hunsdiecker reaction video
REFERENCES
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- Laue T.,Plagens A., Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005






