Table of Contents
ToggleConformation of disubstituted cyclohexane includes two commonly used forms ie chair and boat forms which are interconvertible at ordinary temperatures. Depending upon the position where the substituent is attached, the stability is explained. It can be shown in two forms that are trans and cis. In this specific conformation of disubstituted cyclohexane, we are going to explain the conformer and stability of 1,2-,1,3-, and 1,4 disubstituted cyclohexane and going to find out which one is more stable and which one is the least.
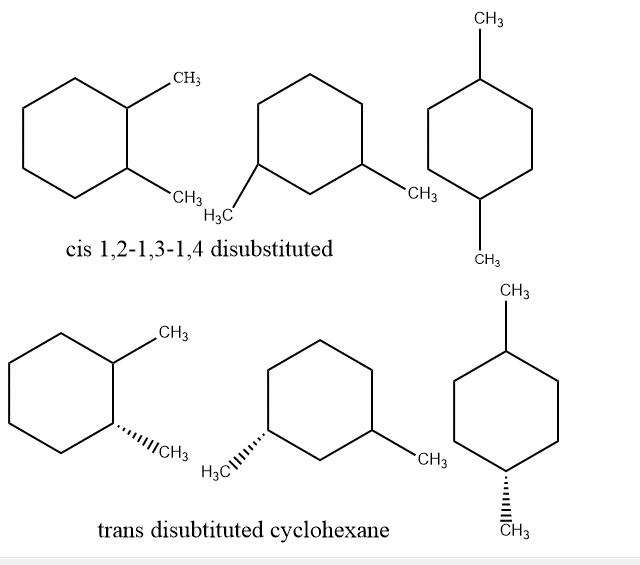
Disubstituted cyclohexane
Generally, the conformation of disubstituted cyclohexane is divided into various positions according to their position of substituents. Starting from 1,1- disubstituted cyclohexane, trans and cis forms are formed as they both have the same substituents and cannot be expressed into two forms.
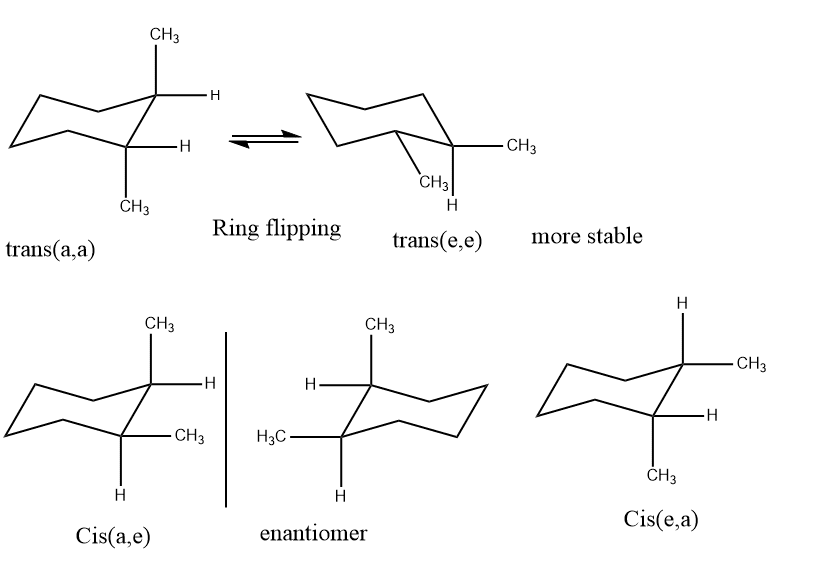
Conformational analysis of 1,2 disubstituted cyclohexane
It has two possible interpretations of this position: trans and cis. Additionally, the substituents are present at positions 1 and 2. Contrarily, if the same substituent is in both axial and axial or equatorial and equatorial, it can be termed trans. If one substituent is equatorial and another is axial or vice versa, it can be considered cis. The trans (e,e) will be more stable and trans(a, a) will be the least stable. The order of stability will be
(e,e)>(e,a)>(a,e)>(a,a)
e,e= No 1,3-diaxial interaction
a,e= two 1,3-diaxial interaction
a,a= four 1,3-diaxial interaction
Due to its two axial methyl groups, the a, a conformer has a steric energy of 3.48 kcal/mol. This indicates that there are four gauche-butane interactions in the diaxial form, which equate to an interaction energy of 3.2 or 3.6, or precisely 3.48 kcal/mole. Since these methyl groups are gauche to one another, the diequatorial form only exhibits one such interaction, which has an energy of 0.8 or 0.9 kcal/mol.
According to the result derived from planar models, the cis-forms a dl pair, or 1,2- disubstituted at the cis position of cyclohexane, is a chiral molecule that exists in enantiomers.
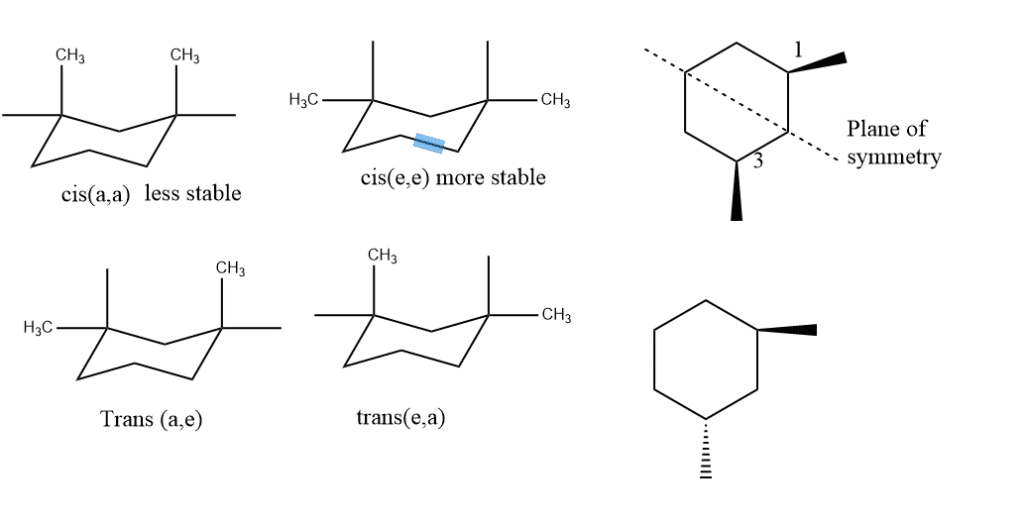
Conformational analysis of 1,3- disubstituted cyclohexane
The situation in 1,3- disubstituted that is 1,3-dimethyl cyclohexane is simpler because both diastereomers exist in a single conformation. In cis form, both the substituents are either equatorial or axial just opposite in the case of 1,2- disubstitution. And in the case of trans the substituents are axial-equatorial or equatorial-axial. In trans-form, the molecule does not have a plane of symmetry, and rings flips occur.
In this case, the most stable form will be cis(e,e) and the least will be cis(a, a) which is just the opposite of 1,2- dimethyl cyclohexane. The relative stabilities depend upon which isomer has the most stable conformer. The stable conformers of 1,3- dimethyl cyclohexane is cis(e,e) form. The preferred chair has both methyl groups equatorial, which minimizes 1,3-diaxial repulsions. Cis form has the plane of symmetry and is achiral and forms a Meso compound.
stability order= ee>ae>aa
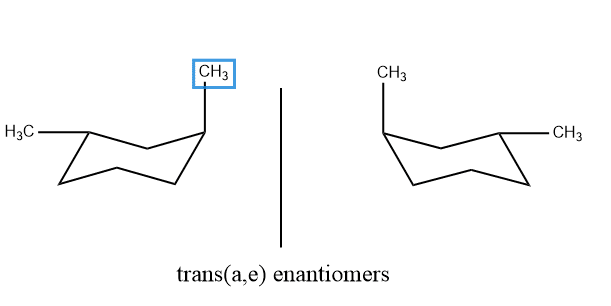
Trans are configurational enantiomers because they are not interconvertible by flipping.
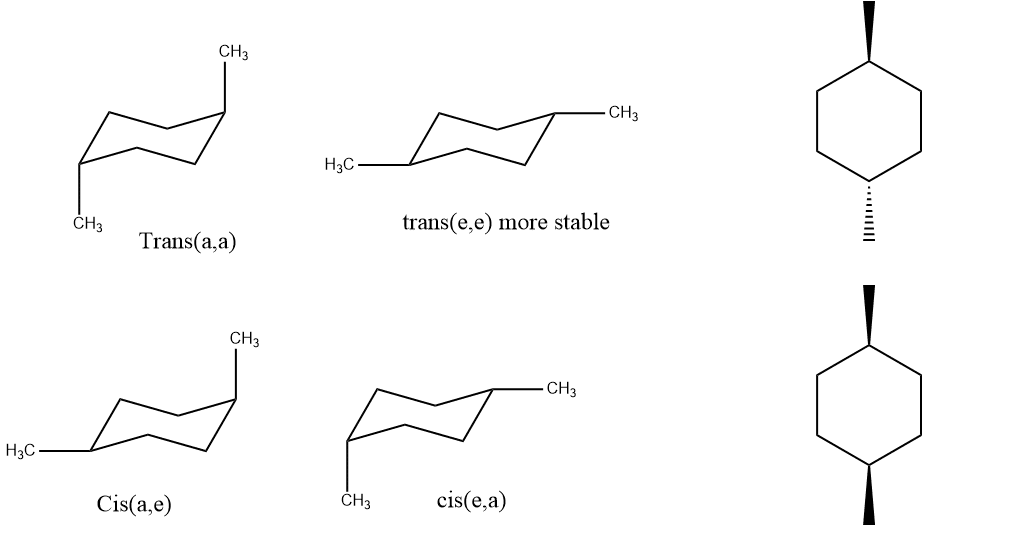
Conformational analysis of 1,4-disubstituted cyclohexane
In the case of 1,4-dimethyl cyclohexane, the stability is the same as 1,2-disubstituted cyclohexane. If the cis form is there, one will be equatorial and another axial or vice versa. And in the case of trans, both will be either equatorial or both will be axial.
The preferred chair has both methyl groups equatorial, which reduces steric repulsions which can be observed in trans(e,e) form which is 7kj/mol more stable than the cis isomers. Neither cis nor trans is chiral because both have a plane of symmetry.
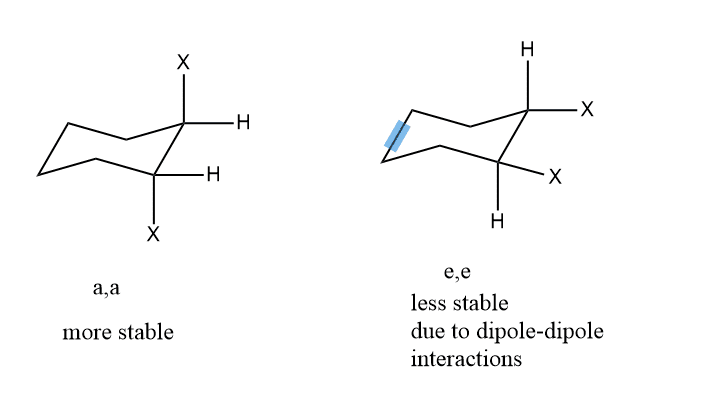
Note: if instead of alkyl groups, halogen or alcohol is present, (a, a) will be more stable because of dipole-dipole repulsion in the (e,e) position.
FAQs/MCQs
Which form of disubstituted cyclohexane is more stable?
The more stable isomer is trans-1,2-dimethyl cyclohexane because it has the most stable conformer.
Which 1,4-disubstituted form is most stable?
In comparison to the cis-1,4-disubstituted cyclohexane conformation, the trans version is more stable. since in this case there are no 1,3-diaxial interactions. It is strain-free and has a lower energy transformation. The conformation becomes more stable as a result.
Which conformation of cyclohexane is destabilized?
Cyclohexane’s boat conformation possesses two different kinds of destabilizing interactions, namely eclipsing and flagpole-flagpole. The most unstable of these is eclipsing interaction.






